Updated Guidelines on Amniocentesis and Chorionic Villus Sampling: Key Recommendations for Safe Prenatal Diagnosis
UK: The Royal College of Obstetricians and Gynaecologists (RCOG) has updated its Green-top Guideline No. 8 on amniocentesis and chorionic villus sampling (CVS), offering evidence-based recommendations for prenatal diagnosis. This fifth edition, updated from the previous version published in June 2010, guides the appropriate use of these procedures for pregnant women at risk of genetic disorders or other pregnancy complications.
The guidelines were published online in BJOG: An International Journal of Obstetrics & Gynaecology.
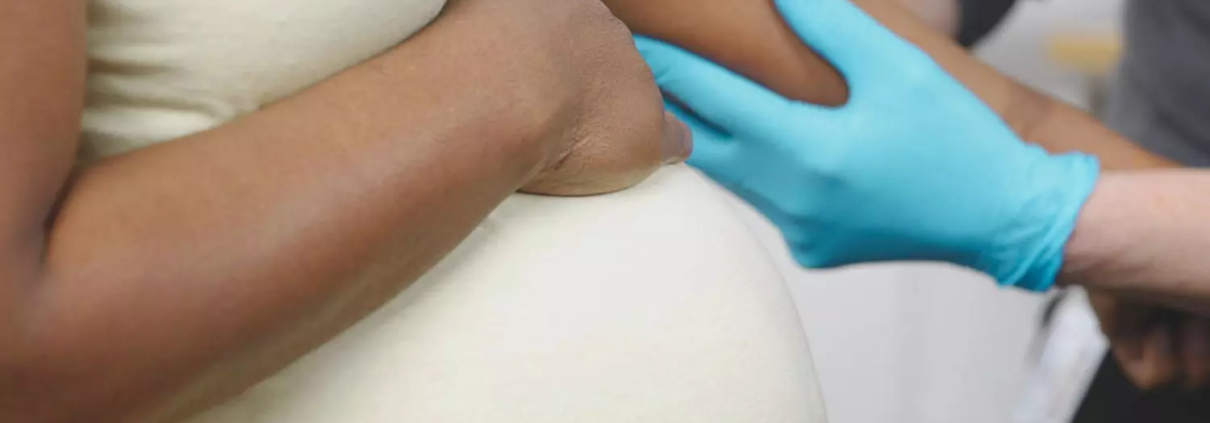
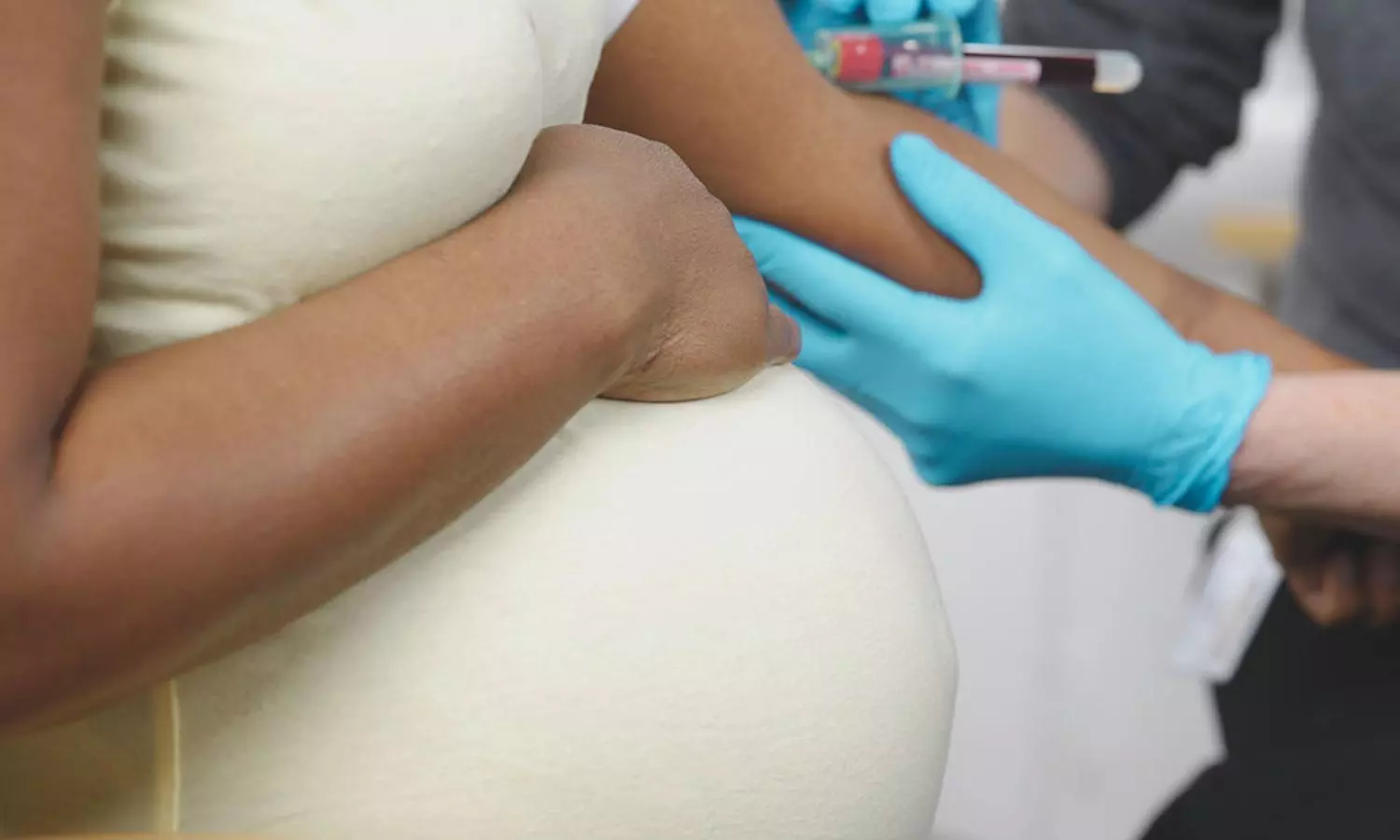
Amniocentesis and CVS are commonly offered to pregnant women for prenatal testing, especially when there is an increased likelihood of genetic conditions such as Down syndrome or when fetal anomalies are detected through screening. CVS is typically performed between 11 and 13 weeks of gestation, although it may be carried out as late as 14+6 weeks if necessary. For those considering CVS during this time, individualized counseling is recommended to weigh the benefits and risks of CVS versus amniocentesis. The latter is usually performed after 15 weeks of gestation to obtain amniotic fluid for analysis.
One of the key points highlighted in the guideline is the importance of informing patients about the risks of miscarriage associated with both procedures. When performed by appropriately trained operators, the risk of miscarriage following amniocentesis or CVS is generally below 0.5%, a reassuring statistic for most women. However, in cases of multiple pregnancies, such as twins, the miscarriage risk is around 1%, so this should be discussed with patients.
The guidelines stress that amniocentesis should not be performed before 15 weeks of gestation, while CVS should not be conducted before 10 weeks. The ideal time for CVS is between 11 and 13 weeks to minimize technical difficulties and ensure better sample collection. The guidelines also include a note on blood-borne viruses, advising that viral load and antigen test results should be reviewed before conducting invasive tests, and discussing the risk of viral transmission.
These updated guidelines ensure that both amniocentesis and CVS are performed safely and effectively, with clear patient counseling about their options. This evidence-based approach supports clinicians and pregnant women in making informed decisions about prenatal genetic testing.
The authors suggest that future research should focus on examining the rates of procedure-related pregnancy loss in multiple pregnancies, particularly considering chorionicity. Additionally, they recommend investigating the risk of mother-to-child transmission following invasive procedures during acute infections, such as Hepatitis C, to better understand the potential complications in these scenarios.
Reference:
Navaratnam, K., & Alfirevic, Z. (2021). Amniocentesis and chorionic villus sampling. BJOG: An International Journal of Obstetrics & Gynaecology, 129(1), e1-e15. https://doi.org/10.1111/1471-0528.16821