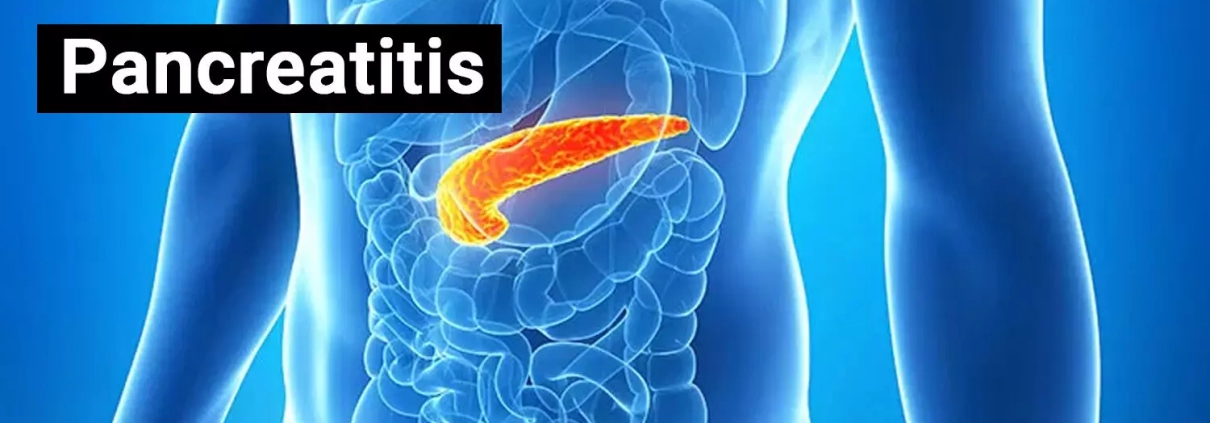
Prophylactic pancreatic stent in addition to rectal indomethacin effective for preventing post-ERCP pancreatitis
Endoscopic retrograde cholangiopancreatography (ERCP) is a valuable diagnostic and therapeutic procedure, but it comes with the risk of pancreatitis, particularly in high-risk patients. A recent randomized trial assessed two preventive strategies—rectal indomethacin alone versus the combination of indomethacin plus a prophylactic pancreatic stent.
This study was published in the journal Lancet by Prof B Joseph E. and colleagues. The study aimed to evaluate efficacy and safety, providing valuable insights for enhancing post-ERCP patient care.Therandomized non-inferiority trial involving 1950 high-risk patients undergoing endoscopic retrograde cholangiopancreatography (ERCP), researchers evaluated the efficacy of two preventive strategies: rectal indomethacin alone versus the combination of indomethacin plus a prophylactic pancreatic stent.
-
The primary outcome, post-ERCP pancreatitis incidence, was 14.9% with indomethacin alone and 11.3% with the combination, resulting in a risk difference of 3.6% (95% CI 0.6–6.6; p=0.18 for non-inferiority).
-
However, a post-hoc analysis revealed the superiority of the combination strategy (p=0.011).
-
Safety outcomes, including serious adverse events, intensive care unit admission, and hospital length of stay, did not significantly differ between the two groups.
The trial concludes that, for high-risk patients undergoing ERCP, the strategy of using rectal indomethacin alone was not as effective as combining indomethacin with prophylactic pancreatic stent placement. These findings advocate for the adoption of a comprehensive approach, emphasizing the role of prophylactic stent placement in addition to indomethacin administration for optimal prevention of post-ERCP pancreatitis in high-risk patients.
Reference: