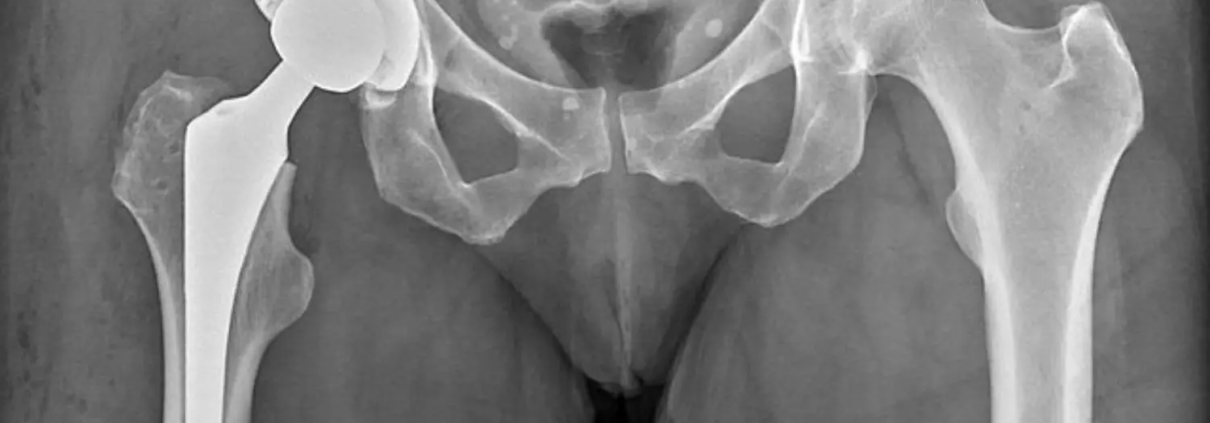
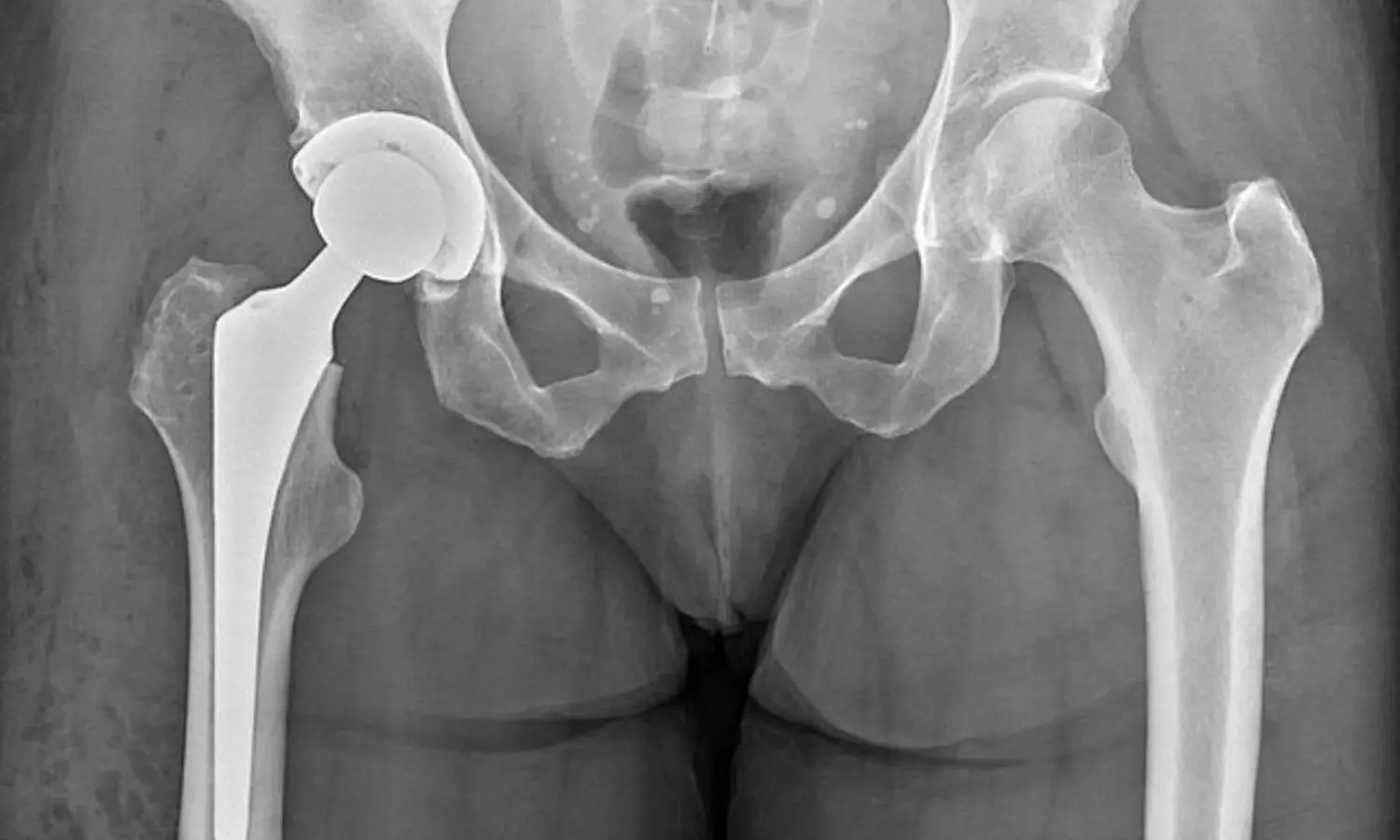
Optimal Pain Management Strategy Unveiled in Total Hip Arthroplasty Study
In a significant stride towards refining postoperative pain management
strategies following total hip arthroplasty, the RECIPE trial has shed light on
an innovative drug combination that showcases promise in reducing morphine
consumption and minimizing adverse events. The trial concluded that for adults
undergoing total hip arthroplasty, the combination of paracetamol, ibuprofen,
and dexamethasone proved to be the most effective in reducing morphine
consumption within the first 24 hours after surgery.
The trial results were published in the journal The Lancet Rheumatology.
Total hip arthroplasty often necessitates multimodal postoperative
analgesia, yet the optimal drug combination has remained uncertain. Hence
researchers conducted a randomized, blinded, and placebo-controlled trial at
nine Danish hospitals to investigate the relative benefits and potential harm
associated with different combinations of paracetamol, ibuprofen, and the
analgesic adjuvant dexamethasone in treating postoperative pain.
The Recipe trial was carried out between March 5, 2020, and Nov 15, 2022,
and a total of 1060 participants were enrolled in the trial. Adults scheduled
for total hip arthroplasty were randomly assigned to receive various
combinations of oral paracetamol 1000mg every 6h, oral ibuprofen 400 mg every 6
h, and a single dose of intravenous dexamethasone 24 mg. The primary outcome assessed was 24-hour intravenous morphine
consumption, with a predefined minimal important difference of 8 mg.
Findings:
-
The results, encompassing a modified intention-to-treat population of
1043 participants, unveiled intriguing findings. -
The combination of paracetamol, ibuprofen, and dexamethasone demonstrated
the lowest 24-hour morphine consumption following surgery. -
Although the differences did not reach the predefined minimal important
threshold of 8 mg, the paracetamol plus ibuprofen plus dexamethasone group
exhibited a significant reduction in morphine consumption compared to other
combinations.
Moreover, the safety profile of this innovative drug combination proved
to be highly favorable.Adverse events were reported in 35% of participants in the paracetamol
plus ibuprofen plus dexamethasone group, showcasing a lower incidence compared
to 38% in the ibuprofen plus dexamethasone group, 39% in the paracetamol plus
dexamethasone group, and a higher incidence of 63% in the paracetamol plus
ibuprofen group.
The adverse events primarily included differences in nausea, vomiting,
and dizziness.
This novel approach to postoperative analgesia holds promise in
revolutionizing pain management strategies for total hip arthroplasty patients.
The combination of paracetamol, ibuprofen, and dexamethasone not only
demonstrated efficacy in reducing morphine consumption but also exhibited a
safer adverse event profile, marking a significant advancement in optimizing
patient care.
As the medical community continues to
explore innovative solutions for postoperative pain relief, the RECIPE trial’s
findings provide a valuable contribution to the ongoing discourse, offering a
potential paradigm shift in enhancing the recovery experience for individuals
undergoing total hip arthroplasty.Top
of Form
Further reading: Steiness J, Hägi-Pedersen D, Lunn TH, et al. Paracetamol, ibuprofen and dexamethasone for pain treatment after total hip arthroplasty: protocol for the randomised, placebo-controlled, parallel 4-group, blinded, multicentre RECIPE trial. BMJ Open. 2022;12(9):e058965. Published 2022 Sep 1. doi:10.1136/bmjopen-2021-058965