Lotilaner Ophthalmic Solution Promising Treatment for Demodex Blepharitis: Study
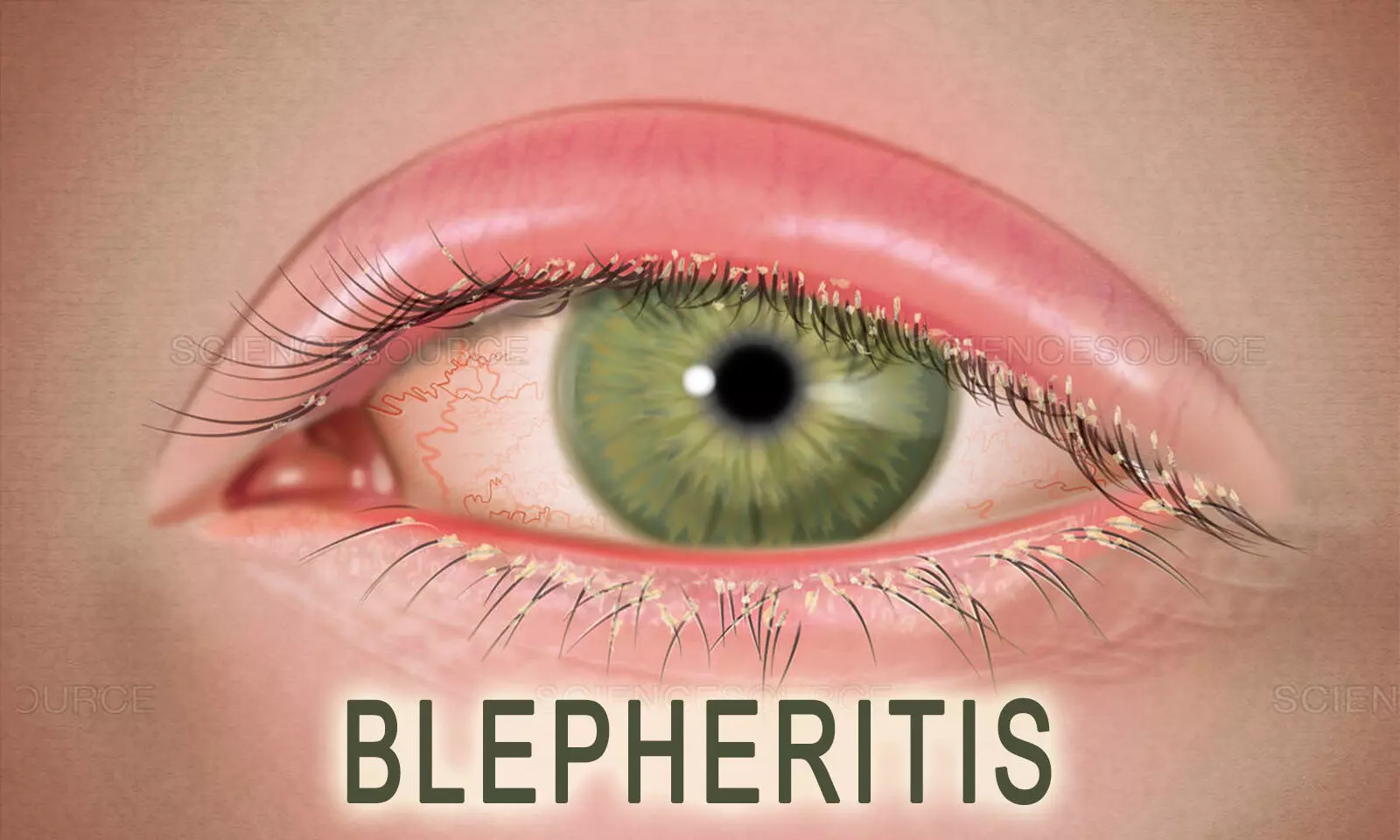
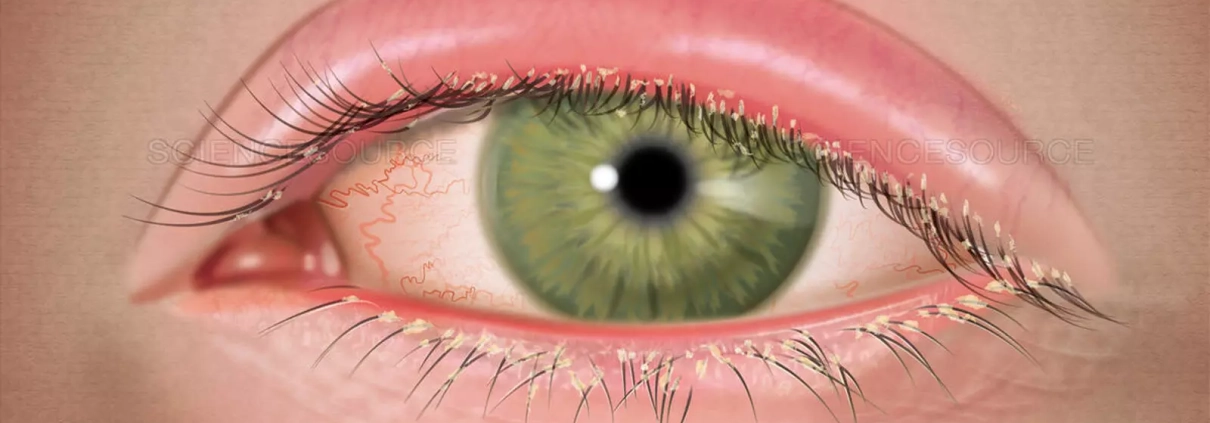
Demodex blepharitis, caused by an infestation of Demodex mites, poses a significant burden on affected individuals, impacting their quality of life. Traditional management options have been associated with adverse effects, prompting the search for novel therapeutic alternatives. A recent systematic review and meta-analysis assessed the safety and efficacy of lotilaner ophthalmic solution (0.25%) as a treatment for Demodex blepharitis. The study was published in the American Journal Of Ophthalmology. The study was conducted by Talha M. and colleagues.
Demodex blepharitis affects millions of individuals worldwide, leading to various ocular manifestations. Prior management strategies, including topical tea tree oil and antibiotics, were associated with adverse effects. Lotilaner, an antiparasitic compound, offers a targeted approach for Demodex eradication.
The systematic review included data from four randomized controlled trials and two single-arm studies, comprising a total of 328 neonates. Studies were selected based on eligibility criteria, including treatment with lotilaner ophthalmic solution and assessment of Demodex mite eradication and collarette score.
The key findings of the study were:
-
Effectiveness of Lotilaner:
Lotilaner ophthalmic solution demonstrated efficacy in eradicating Demodex mites compared to the control group (RR, 3.55; 95% CI, 2.87 – 4.40; P < .00001).
The intervention cohort showed a significantly improved clinically meaningful collarette score compared to the control cohort (RR, 3.15; 95% CI, 2.56 – 3.89; P < .00001).
-
Safety Profile:
Rare adverse effects, including mild burning, redness, and blurriness, were reported with lotilaner treatment, reaffirming its favourable safety profile.
Lotilaner ophthalmic solution (0.25%) emerges as a promising treatment option for Demodex blepharitis, offering efficacy in mite eradication and improvement in collarette scores. The findings underscore its potential as a specific and well-tolerated therapy, enhancing the management of this common ocular condition.
The approval of lotilaner by the FDA signifies a significant advancement in the management of Demodex blepharitis. Its targeted mechanism of action and favourable safety profile position it as a valuable addition to existing treatment strategies, benefiting patients and healthcare providers alike.
Reference:
Talha M, Haris Ali M, Fatima E, Nadeem A, Ahmed A, Nashwan AJ. Efficacy and Safety of Lotilaner Ophthalmic Solution (0.25%) for the Treatment of Demodex Blepharitis: A GRADE Assessed Systematic Review and Meta-Analysis of Observational & Experimental Studies. Am J Ophthalmol. Published online March 19, 2024. doi:10.1016/j.ajo.2024.03.019