Gut-skin connection is key factor in atopic dermatitis, research review shows
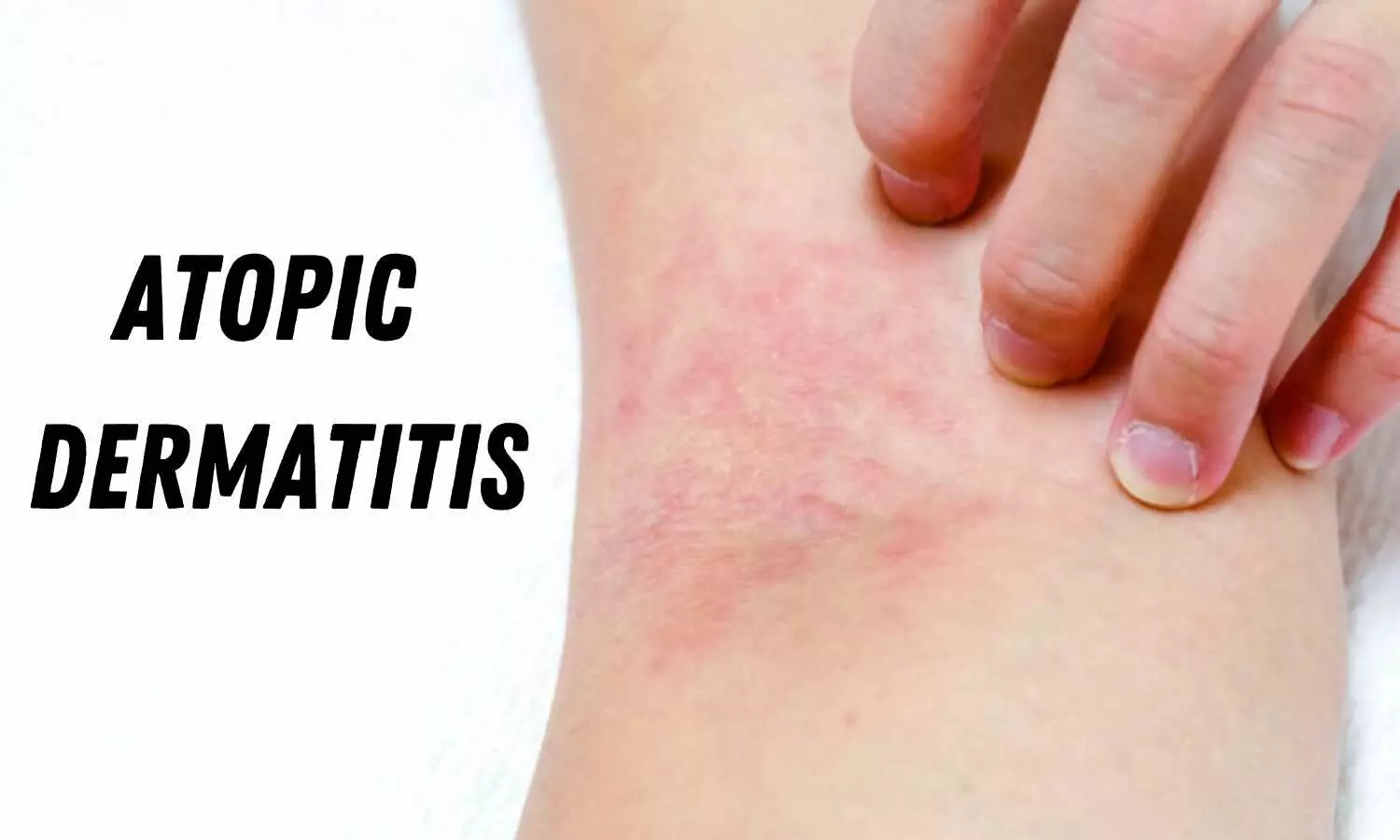
Atopic dermatitis (AD) is a chronic inflammatory skin disease whose main symptoms are redness, swelling, and itchy rashes. It is more common in people with a genetic predisposition. Manifestation of symptoms depends on interactions among the immune system, environmental factors and gut microbiota. Many pieces of this complex jigsaw puzzle are still undiscovered, but recent research has identified relevant factors. For example, alterations in gut microbiota composition can contribute to the severity of the disease; environmental factors such as allergens and pollution can also make it worse; genetic variations are associated with susceptibility; and both diet and fecal transplantation are promising strategies for treatment.
Knowing how these factors correlate is fundamental to a better understanding of the disease and serves as a basis for novel therapies, according to a review article published in the International Journal of Molecular Sciences by researchers at the University of São Paulo (USP) and the Federal University de São Paulo (UNIFESP) in Brazil.
Also known as atopic eczema, AD affects 7%-10% of adults and 20%-25% of young children. There is no consensus as to whether boys or girls are more affected. The number of cases has increased significantly in the twenty-first century. Scientists believe the rise is due to several factors, such as genetics, autoimmunity, impaired skin barrier integrity, viral infections, gut microbiome composition, dietary habits and lifestyle changes.
A hypothesis that has been proposed to explain the significant increase in developing countries is lack of exposure to beneficial bacteria, which may affect immune maturation (the process by which the immune system develops a response after first contact with microorganisms).
Importance of gut microbiota
The authors of the review, which was supported by FAPESP, show that gut microbiota is at the center of the most recent research. “Besides being responsible for 70% of immune system regularization, for maintaining skin barrier integrity and the structure of the gastrointestinal tract, and for controlling nutrient absorption and energy balance, the gut microbiome is directly connected to the skin via what’s known as the gut-skin axis,” said Sabri Saeed Sanabani, a researcher at the Institute of Tropical Medicine (IMT-USP) and last author of the article.
The article features recent evidence that alterations to gut microbiome composition can contribute to the pathogenesis of AD. Studies have reported increased abundance of Clostridium difficile, Escherichia coli and Staphylococcus aureus, as well as decreased abundance of bacteria that produce short-chain fatty acid (SCFAs), such as Bifidobacteria and Bacteroides, in the gut microbiome of AD patients compared to healthy controls. A reduction in levels of SCFAs is often associated with intestinal inflammation in otherwise healthy subjects.
With regard to genetics, the latest research on the subject involves genome-wide association studies (GWAS), focusing on the identification of associations between genetic variants and important phenotypes, and has so far found several markers that correlate with susceptibility to AD and its progression, including mutations in the filaggrin gene (FLG) that are the most well-established risk factor for AD. Filaggrin (a portmanteau for filament aggregating protein) is a protein that binds to keratin fibers in epithelial cells. Whether gut microbiome alterations are genetically determined is unknown, however.
While environmental factors also remain mostly unknown, scientists are certain that allergens, irritants, pollution and exposure to microbes contribute to skin barrier impairment and gut microbiome dysbiosis.
The review also covers promising therapeutic approaches, such as those targeting epigenetic alterations and modulation of changes to gut microbiome diversity via diet, probiotics and prebiotics, as well as fecal transplants.
“Like all such reviews, ours set out to analyze the findings of the available scientific studies and verify knowledge gaps that need to be filled by future research,” Sanabani said.
Reference:
Pessôa, R.; Clissa, P.B.; Sanabani, S.S. The Interaction between the Host Genome, Epigenome, and the Gut–Skin Axis Microbiome in Atopic Dermatitis. Int. J. Mol. Sci. 2023, 24, 14322. https://doi.org/10.3390/ijms241814322.