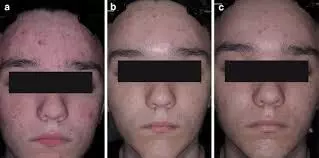
For Moderate to Severe acne, CAB gel turns out to be effective treatment. Study
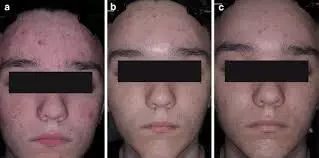
For Moderate to Severe acne, CAB gel turns out to be effective treatment suggests a study published in the Journal of Drugs in Dermatology.
Clindamycin phosphate 1.2%/adapalene 0.15%/benzoyl peroxide 3.1% gel (CAB) is the only fixed-dose triple-combination treatment approved for acne. This post hoc analysis assessed the impact of sex on efficacy and safety/tolerability of CAB.
Methods: In two multicenter, double-blind, phase 3 studies (NCT04214639 and NCT04214652), participants aged ≥9 years with moderate-to-severe acne were randomized (2:1) to 12 weeks of once-daily treatment with CAB or vehicle gel. Pooled data were analyzed by sex. Assessments included treatment success (≥2-grade reduction from baseline in Evaluator’s Global Severity Score and a score of 0 [clear] or 1 [almost clear]), inflammatory/noninflammatory lesion counts, Acne-Specific Quality of Life (Acne-QoL) questionnaire, treatment-emergent adverse events (TEAEs), and cutaneous safety/tolerability. Results: At week 12, treatment success rates were significantly greater with CAB versus vehicle irrespective of sex (females: 53.7% vs 23.0%; males: 43.1% vs 24.6%; P<0.05, both). CAB-treated female and male participants both experienced greater reductions from baseline versus vehicle in inflammatory (females: 77.7% vs 57.9%; males: 77.5% vs 57.1%; P<0.001, both) and noninflammatory lesions (females: 72.5% vs 45.6%; males: 72.3% vs 49.6%; P<0.001, both). Acne-QoL improvements from baseline to week 12 were significantly greater with CAB than vehicle. No significant differences in any efficacy measures between CAB-treated males and females were observed. Most TEAEs were of mild-to-moderate severity; no sex-based trends for safety/tolerability were observed. CAB demonstrated comparable efficacy, quality-of-life improvements, and safety in female and male participants with moderate-to-severe acne. As the first fixed-dose, triple-combination topical formulation, CAB represents an important new treatment for acne.
Reference:
Lain ET, Bhatia N, Kircik L, Gold LS, Harper JC, Bunick CG, Guenin E, Baldwin H, Feldman SR, Rosso JQD. Clindamycin Phosphate 1.2%/Adapalene 0.15%/Benzoyl Peroxide 3.1% Gel for Male and Female Acne: Phase 3 Analysis. J Drugs Dermatol. 2024 Oct 1;23(10):873-881. doi: 10.36849/JDD.2024.8484. PMID: 39361705.
Keywords:
For, Moderate, Severe, acne, CAB, gel, turns, effective, treatment, study, Lain ET, Bhatia N, Kircik L, Gold LS, Harper JC, Bunick CG, Guenin E, Baldwin H, Feldman SR, Rosso JQD, Journal of Drugs in Dermatology