FDA approves red light LED lamp for patients with actinic keratosis
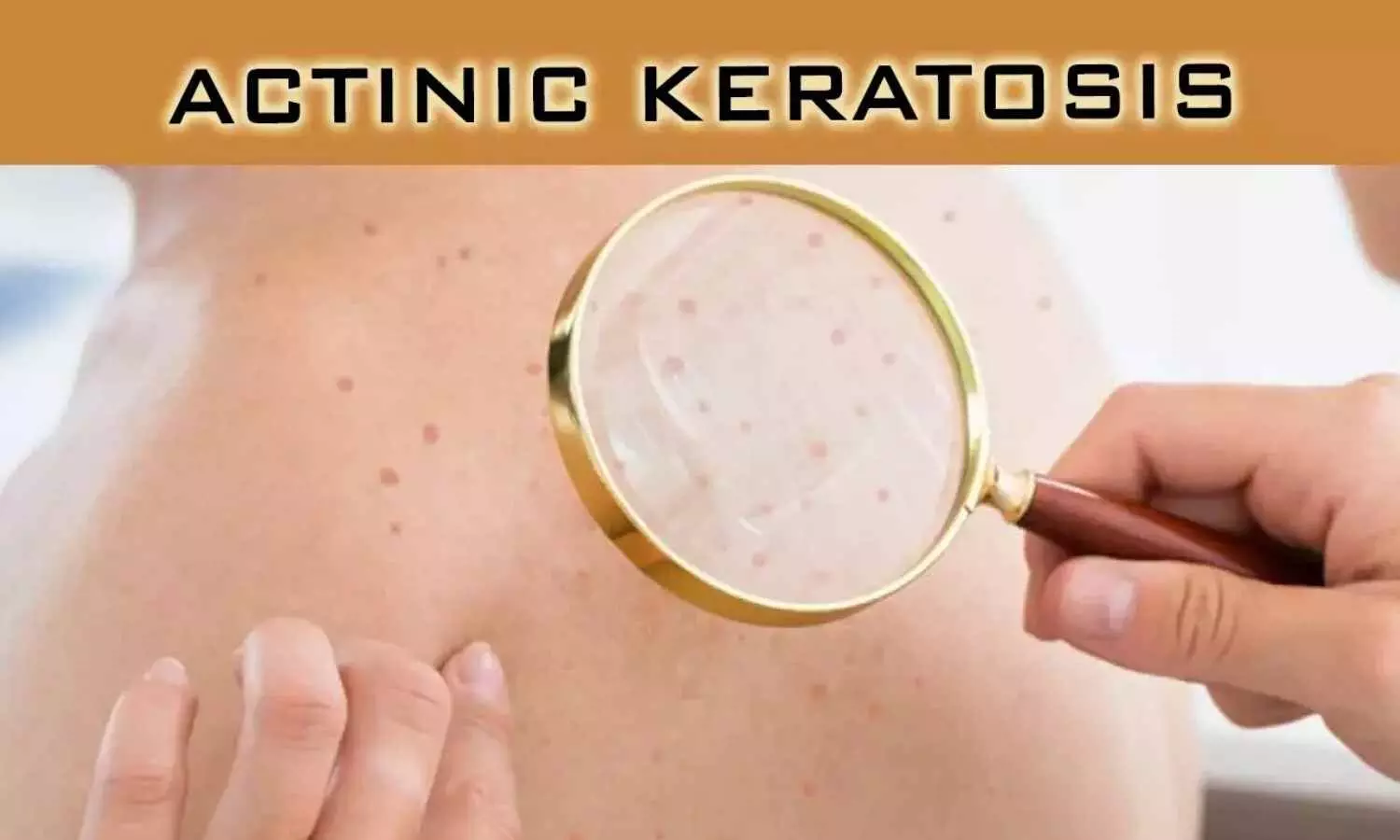
The US food and Drug Administration has approved red light LED lamp for patients with actinic keratosis.The new device was is recommend to be used in combination with aminolevulinic acid hydrochloride topical gel, 10% (Ameluz) as photodynamic therapy (PDT) for individuals with actinic keratoses of mild-to-moderate severity on the face and scalp.
This innovative device represents a significant advancement in the treatment of PDT with state-of-the-art engineering, robust but sleek construction and an intuitive user interface. It is designed to be simple to maneuver and able to accommodate various patient treatment positions in order to optimize ease of use.
Biofrontera’s PDT drug, Ameluz®, is approved by the FDA in combination with either member of the RhodoLED lamp family. The introduction of the RhodoLED XL provides the option to illuminate a larger area in a single on-label Ameluz PDT treatment.
“We are delighted to introduce the RhodoLED XL lamp to the dermatology community in the US,” said Dr. Hermann Luebbert, CEO of Biofrontera Inc. “This new device underscores our commitment to innovation and excellence, providing dermatologists with a powerful tool to deliver superior patient care. The XL lamp’s advanced features and user-friendly design will undoubtedly set a new standard in photodynamic therapy.”
In comparison to Biofrontera’s existing lamp, the RhodoLED XL offers a larger illumination area with five panels compared to one, and additional features such as positioning sensors to ensure the patient receives the optimal energy delivery from the LED array.
Dr. Luebbert continued “I’m pleased to say we have already shipped three of these new devices to customers in our first week of launch and the response from dermatology organizations across the US has been very encouraging.”
Cleaver Dermatology, a renowned practice with offices in Missouri and Georgia, was the first to have the RhodoLED XL lamp installed.
“We are honored to be the first to integrate the XL lamp into our practice,” said Dr. David Cleaver. “The advanced features and versatility of this device represent a considerable upgrade on what has been available until now and will allow us to provide our patients with the highest standard of care. In addition, we have been very pleased with the level of customer service that the team at Biofrontera has delivered.”