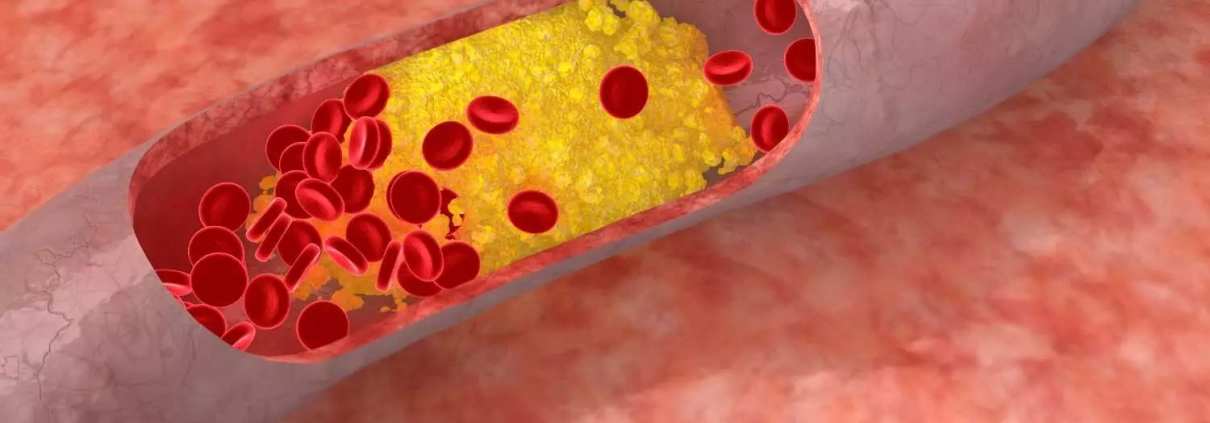
Extended use of inclisiran safe and efficacious among refractory dyslipidemia in patients with increased CV risk
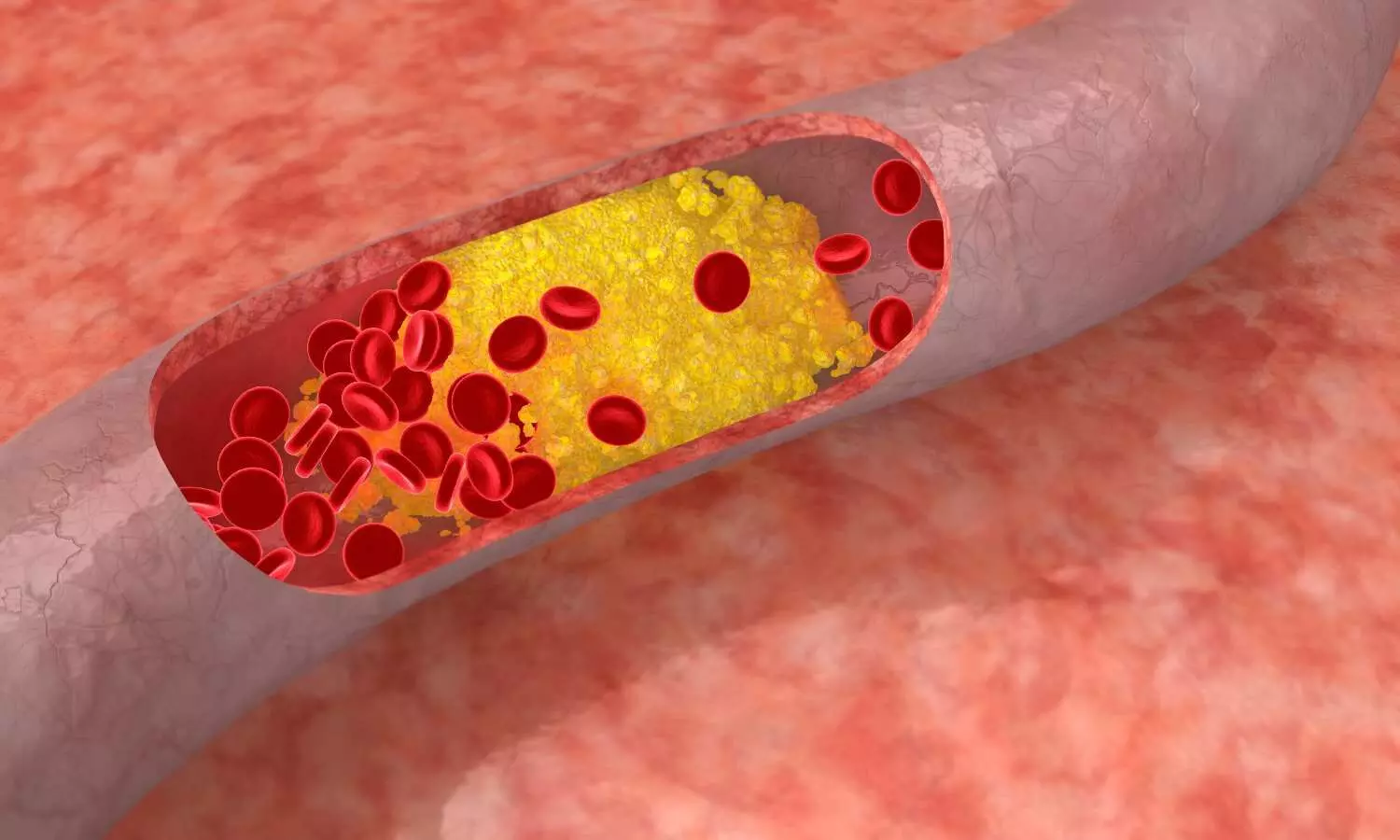
A recent study sheds light on the safety profile of inclisiran, an innovative agent designed to reduce low-density lipoprotein cholesterol (LDL-C). The research aimed to provide comprehensive evidence on the long-term safety of inclisiran, a small interfering RNA agent with promising implications for managing dyslipidemia. This study was published in the Journal Of The American College Of Cardiology by Scott Wright and colleagues.
The post hoc analysis encompassed patients treated with 300 mg inclisiran sodium or placebo across completed (ORION-1, -3, -5, -9, -10, and -11) and ongoing (ORION-8) trials. Key findings from the study:
-
Patient Cohort: The analysis involved 3,576 patients receiving inclisiran for up to 6 years and 1,968 patients on placebo for up to 1.5 years, accounting for 9,982.1 and 2,647.7 patient-years of exposure, respectively. Baseline characteristics were similar across groups, ensuring a balanced comparison.
-
Safety Assessment: Kaplan-Meier analyses revealed that treatment-emergent adverse events (TEAEs), particularly serious ones or those leading to discontinuation, as well as hepatic, muscle, and kidney-related events, exhibited comparable rates between inclisiran and placebo groups for up to 1.5 years. Notably, trends continued favorably for inclisiran beyond this timeframe.
-
Cardiovascular Events: Fewer major cardiovascular events were reported as TEAEs with inclisiran during the observed period, hinting at potential cardiovascular benefits beyond cholesterol reduction.
-
Antidrug Antibodies: Treatment-induced antidrug antibodies were infrequent with inclisiran, reported in 4.6% of cases, with only a small fraction showing persistence (1.4%). Importantly, the presence of these antibodies did not associate with a higher incidence of TEAEs leading to drug discontinuation or serious TEAEs.
The study’s conclusive findings emphasize the favorable long-term safety profile of inclisiran in a diverse patient population with dyslipidemia. This robust evidence underscores the absence of new safety concerns and reiterates the promising safety profile of inclisiran as a therapeutic option for managing cholesterol levels.
The study’s comprehensive analysis, spanning multiple trials and years of patient data, provides valuable insights into inclisiran’s safety landscape. These findings contribute significantly to validating inclisiran’s safety, fostering confidence in its use for patients grappling with dyslipidemia.
Reference:
Wright, R. S., Koenig, W., Landmesser, U., Leiter, L. A., Raal, F. J., Schwartz, G. G., Lesogor, A., Maheux, P., Stratz, C., Zang, X., & Ray, K. K. Safety and tolerability of inclisiran for treatment of hypercholesterolemia in 7 clinical trials. Journal of the American College of Cardiology,2023;82(24):2251–2261. https://doi.org/10.1016/j.jacc.2023.10.007