Epinephrine sublingual film found effective for treatment of severe allergic reactions in topline pharmacokinetic data

Aquestive Therapeutics, Inc. released positive topline PK data from the self-administration study of Anaphylm™ (epinephrine) Sublingual Film.
According to topline pharmacokinetic data released by Aquestive Therapeuticsregarding an epinephrine sublingual film if approved could become the first non-invasive and orally-administered epinephrine treatment for individuals with severe allergic reactions such as anaphylaxis.
Anaphylm has the potential to be the first and only non-invasive, orally delivered epinephrine for the treatment of severe life-threatening allergic reactions, including anaphylaxis, if approved by the United States Food and Drug Administration (FDA).
“The self-administration data again demonstrates the versatility of Anaphylm, as a product that is easy to remember, easy to carry, and easy to use,” said Daniel Barber, President & Chief Executive Officer of Aquestive. “Our groundbreaking Anaphylm formulation indicates that rapid and substantial epinephrine absorption is achieved under a variety of administration conditions. This built-in functionality addresses potential real-world emergency scenarios, where ideal administration may not happen. In contrast to single-use medical devices, Anaphylm has unique administration properties that allow delivery of the needed levels of epinephrine to provide life-saving medication to patients.”
The single-dose, three-period, randomized crossover study design compared the PK and pharmacodynamics (PD) of Anaphylm self-administered, Anaphylm HCP administered, and Adrenalin manual intramuscular (IM) injection HCP administered. The primary PK parameters were the maximum amount of epinephrine measured in plasma (Cmax) and exposure, or the area under the curve (AUC), at various times after dosing in 36 healthy adult subjects. Graph 1 below provides a comparison of epinephrine concentration across the first 60 minutes post-administration. There was no statistical difference between the Anaphylm self-administered and HCP-administered arms of the study. The median time to maximum concentration (Tmax) was 15 minutes for both the Anaphylm self-administered and HCP-administered arms, while the median Tmax for the Adrenalin IM HCP administered arm was 50 minutes post administration.
Graph 1: Baseline-Corrected Epinephrine Concentration Across Time*:
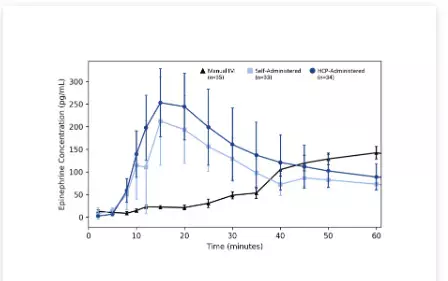
(*Lines on the graph above represent the geometric means of baseline-corrected epinephrine concentration across study timepoints. Baseline-corrected values were calculated by subtracting from the mean of three pre-dose concentrations measured at 60-, 30- and 15-minutes prior to treatment administration.)
“Experiencing and managing a severe allergic reaction can be unsettling and chaotic for patients and caregivers,” said Matthew Greenhawt, MD, MBA, MSc, an anaphylaxis expert, and allergist at Children’s Hospital Colorado and Aquestive Scientific Advisory Board member. “An orally administered product that can be rapidly and easily administered has the potential to be a game-changer for the allergy community. Anaphylm encompasses many features important to patients and caregivers, including ease of carry, ease of administration, rapid delivery of epinephrine, and no needles.”
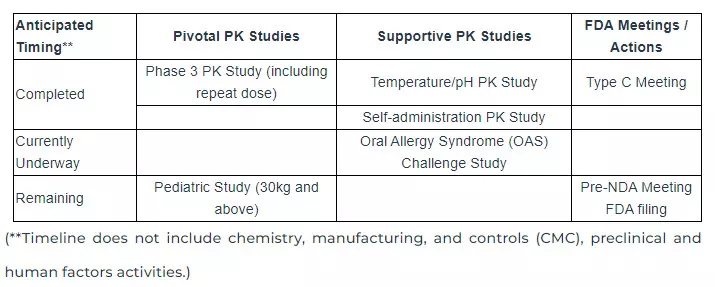
The Company’s remaining supportive study, the oral allergy syndrome (OAS) challenge study, is underway, and the study is expected to be completed late in the third quarter or early fourth quarter of 2024. The Company is maintaining its guidance on a full product launch of Anaphylm at the end of 2025 or in the first quarter of 2026. This is based on filing an NDA late in the fourth quarter of 2024 or early in the first quarter of 2025. The table below indicates the remaining clinical studies anticipated before the submission of the NDA.
About Anaphylaxis
Anaphylaxis is a serious systemic hypersensitivity reaction that is rapid in onset and potentially fatal. As many as 49 million people in the United States are at chronic risk for anaphylaxis. Lifetime prevalence is at least 5%, or more than 16 million people in the United States. Direct costs of anaphylaxis have been estimated at $1.2 billion per year, with direct expenditures of $294 million for epinephrine, and indirect costs of $609 million. The frequency of hospital admissions for anaphylaxis has increased 500–700% in the last 10–15 years. Of patients who previously experienced anaphylaxis, 52% had never received an epinephrine auto-injector prescription, and 60% did not have an auto-injector currently available. The most common causes of anaphylaxis are foods (such as peanuts), venom from insect stings, and medications. Epinephrine injection is the current standard of treatment intended to reverse the severe manifestation of anaphylaxis, which may include skin rash, throat swelling, respiratory difficulty, gastrointestinal distress, and loss of consciousness.
About Anaphylm™
Anaphylm™ (epinephrine) Sublingual Film has the potential to be the first and only non-invasive, orally delivered epinephrine for the treatment of severe life-threatening allergic reactions, including anaphylaxis, if approved by the FDA. Anaphylm is a polymer matrix-based epinephrine prodrug candidate product. The product is similar in size to a postage stamp, weighs less than an ounce, and begins to dissolve on contact. No water or swallowing is required for administration. The packaging for Anaphylm is thinner and smaller than an average credit card, can be carried in a pocket, and is designed to withstand weather excursions such as exposure to rain and/or sunlight. The “Anaphylm” tradename for AQST-109 has been conditionally approved by the FDA. Final approval of the Anaphylm proprietary name is conditioned on FDA approval of the product candidate.