Crisaborole may improve signs and symptoms of Stasis Dermatitis,reveals study
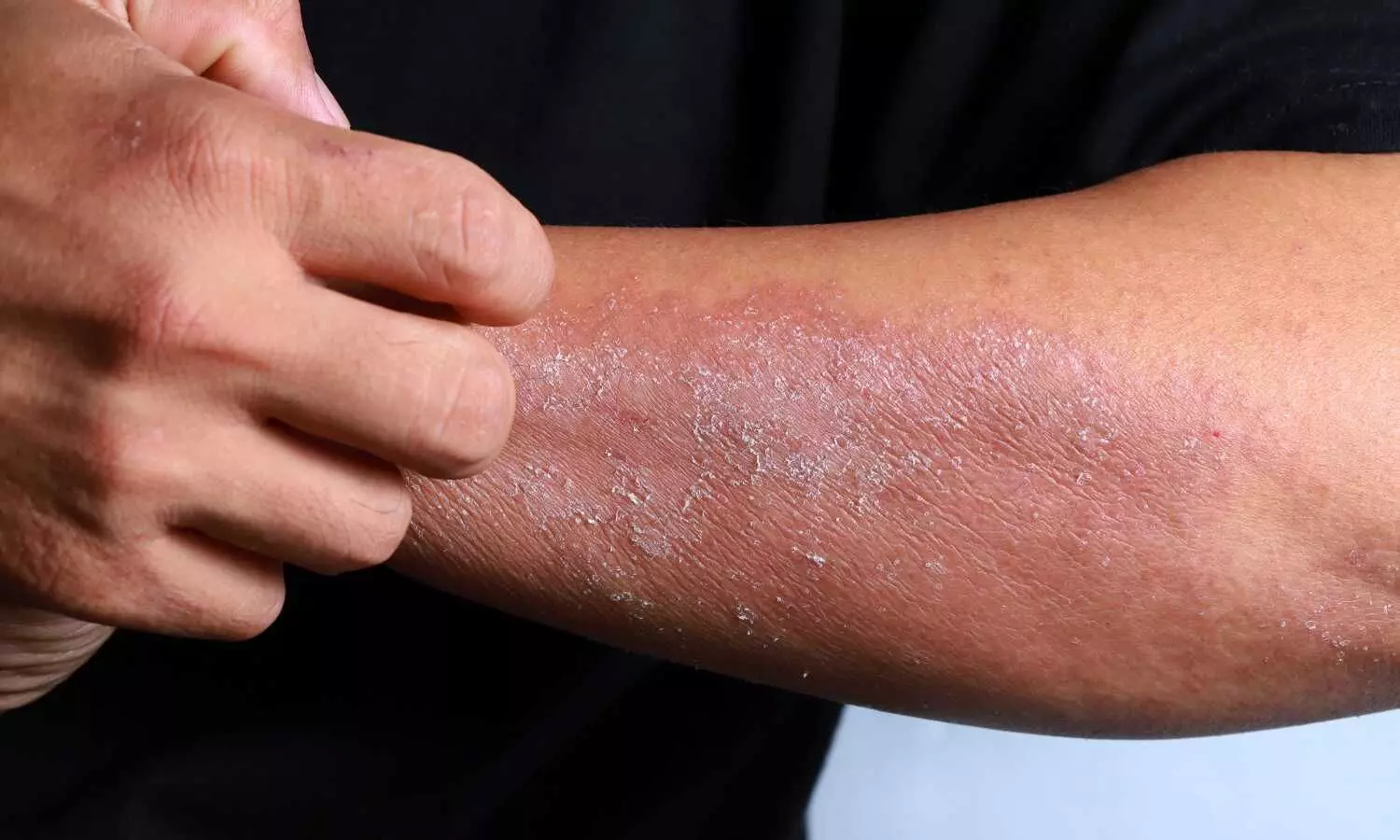
Stasis Dermatitis (SD) is often associated with poor venous circulation and can lead to complications such as skin ulcers and infection. Treatment options for SD are limited, and there is a need for effective and well-tolerated therapies. Crisaborole’s anti-inflammatory properties make it a potential candidate for the management of SD.
Stasis dermatitis (SD) is a common inflammatory skin condition affecting individuals aged 45 years and older, characterized by itching, redness, and swelling. Crisaborole ointment, 2%, a nonsteroidal topical phosphodiesterase 4 inhibitor, has been approved for the treatment of mild-to-moderate atopic dermatitis.
A recent phase 2a study aimed to assess the efficacy and safety of crisaborole in patients with SD. This study was published in the Journal Of The American Academy Of Dermatology by Jonathan I. and colleagues. The randomized, double-blind, vehicle-controlled study enrolled 65 participants with SD without active ulceration. Patients received either crisaborole or vehicle ointment twice daily for 6 weeks. The primary endpoint was the percentage change from baseline in total sign score at week 6, assessed by in-person and central reader evaluations.
The key findings of the study were:
-
Crisaborole-treated participants showed a significant reduction in total sign score from baseline compared to the vehicle group.
-
In-person assessment by nondermatologists demonstrated a reduction of 32.4% in the crisaborole group versus 18.1% in the vehicle group (P = .0299).
-
Central reader assessment of photographs showed an even greater reduction of 52.5% versus 10.3% (P = .0004).
-
Efficacy outcomes, including success and improvement per Investigator’s Global Assessment score and lesional percentage body surface area, reached statistical significance based on central reader assessments but not in-person evaluations.
-
Skin and subcutaneous tissue disorders were the most common treatment-emergent adverse events with crisaborole, indicating that the treatment was generally well tolerated.
Crisaborole ointment, 2%, demonstrated efficacy in improving signs and symptoms of SD in patients aged 45 years and older. The study findings support the use of crisaborole as a potential treatment option for SD. Central reader assessment showed promise as a method for evaluating treatment efficacy in decentralized clinical research settings.
Reference:
Silverberg, J. I., Kirsner, R. S., Margolis, D. J., Tharp, M., Myers, D. E., Annis, K., Graham, D., Zang, C., Vlahos, B. L., & Sanders, P. Efficacy and safety of crisaborole ointment, 2%, in participants aged ≥45 years with stasis dermatitis: Results from a fully decentralized, randomized, proof-of-concept phase 2a study. Journal of the American Academy of Dermatology,2024. https://doi.org/10.1016/j.jaad.2023.12.048


