Study identifies new method for improving lung growth and function in preterm infants
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico
Darbepoetin alfa significantly reduces the reliance on blood transfusions while boosting hemoglobin levels in cancer patients experiencing chemotherapy-induced anemia, a recent Indian study has reported.
A recent real-world retrospective study published in Frontiers in Oncology was conducted at Narayana Super-specialty Hospital-Howrah, a tertiary care center in Kolkata, India; enrolled 523 patients with advanced or metastatic solid tumors undergoing palliative myelosuppressive therapy over a six-year period.
The study analyzed electronic health records to evaluate the impact of darbepoetin alfa (Cresp®, 200 mcg subcutaneous injection by Dr Reddy’s Laboratories, administered biweekly) on chemotherapy-induced anemia. The study included adults (≥18 years) with advanced or metastatic solid tumors who had completed at least two cycles of palliative myelosuppressive chemotherapy. Eligible patients had an Hb level of ≤10.0 g/dL at diagnosis, though those with Hb levels between 10.0 and 11.0 g/dL were included if symptomatic for anemia. Enrollment required at least one dose of darbepoetin alfa. The study excluded pediatric patients, those with early-stage solid tumors receiving curative treatment, individuals with hematological malignancies, patients on targeted therapy alone or with non-myelosuppressive agents, and those previously exposed to recombinant erythropoietin.
The primary endpoint of the study was to measure the change in hemoglobin levels from baseline to post-treatment. Secondary endpoints included the reduction in blood transfusion dependence, improvements in patient-reported anemia symptoms such as fatigue and dyspnea, and the occurrence of treatment-emergent adverse events (TEAEs) such as hypertension, deep vein thrombosis (DVT), and arrhythmias.
The median age of the cohort was 55 years, and patients were divided based on cancer type, chemotherapy regimen, treatment response, and administered doses of darbepoetin alfa.
Key findings of the study include:
Significant Hemoglobin Improvement: Patients exhibited a marked increase in mean hemoglobin levels from a baseline of 8.56 ± 0.45 g/dL to 10.84 ± 0.92 g/dL resulting in a statistically significant 2.28 g/dL rise (P<.001). The benefit of darbepoetin alfa was observed consistently across all analyzed subgroups, including different cancer types (gastrointestinal, head and neck, lung, breast, genitourinary, gynecological malignancies, and others), chemotherapy types (single-agent and combination regimens), chemotherapy protocols (taxane + platinum, gemcitabine + platinum, fluoropyrimidine-based, single-agent taxane, platinum monotherapy, and others). Figure 1.
Change in Hemoglobin in Relation to Cancer Therapy Response: A subgroup analysis revealed that patients demonstrating a partial response to cancer therapy experienced the highest hemoglobin increment (2.66 ± 0.51 g/dL), compared to those with stable disease (1.86 ± 0.82 g/dL) or progressive disease (1.40 ± 0.59 g/dL). Furthermore, patients receiving more than four doses of Darbepoetin alfa showed a superior hemoglobin improvement compared to those administered only four or fewer doses.
Reduction in Blood Transfusion Dependence: At baseline, 50 patients (9.6%) required blood transfusion. Following darbepoetin alfa administration, only 22 patients continued to need transfusions, corresponding to a risk reduction of 56%.
Improvement in Anemia-Related Symptoms: Patient-reported outcomes highlighted significant alleviation in symptoms such as fatigue and dyspnea (P<.001 for both), indicating the quality-of-life benefits linked to improved hemoglobin levels after darbepoetin alfa treatment.
Favorable Tolerability Profile: Adverse events were relatively infrequent. Hypertension (5.4%), deep vein thrombosis (2.9%), and arrhythmias (0.8%) were the most commonly observed treatment-emergent adverse events.
Given the risks of frequent blood transfusions, such as thromboembolic events and immune complications in palliative cancer care, this study demonstrated that erythropoiesis-stimulating agents (ESAs) like Darbepoetin alfa play a critical role in managing chemotherapy-induced anemia (CIA).
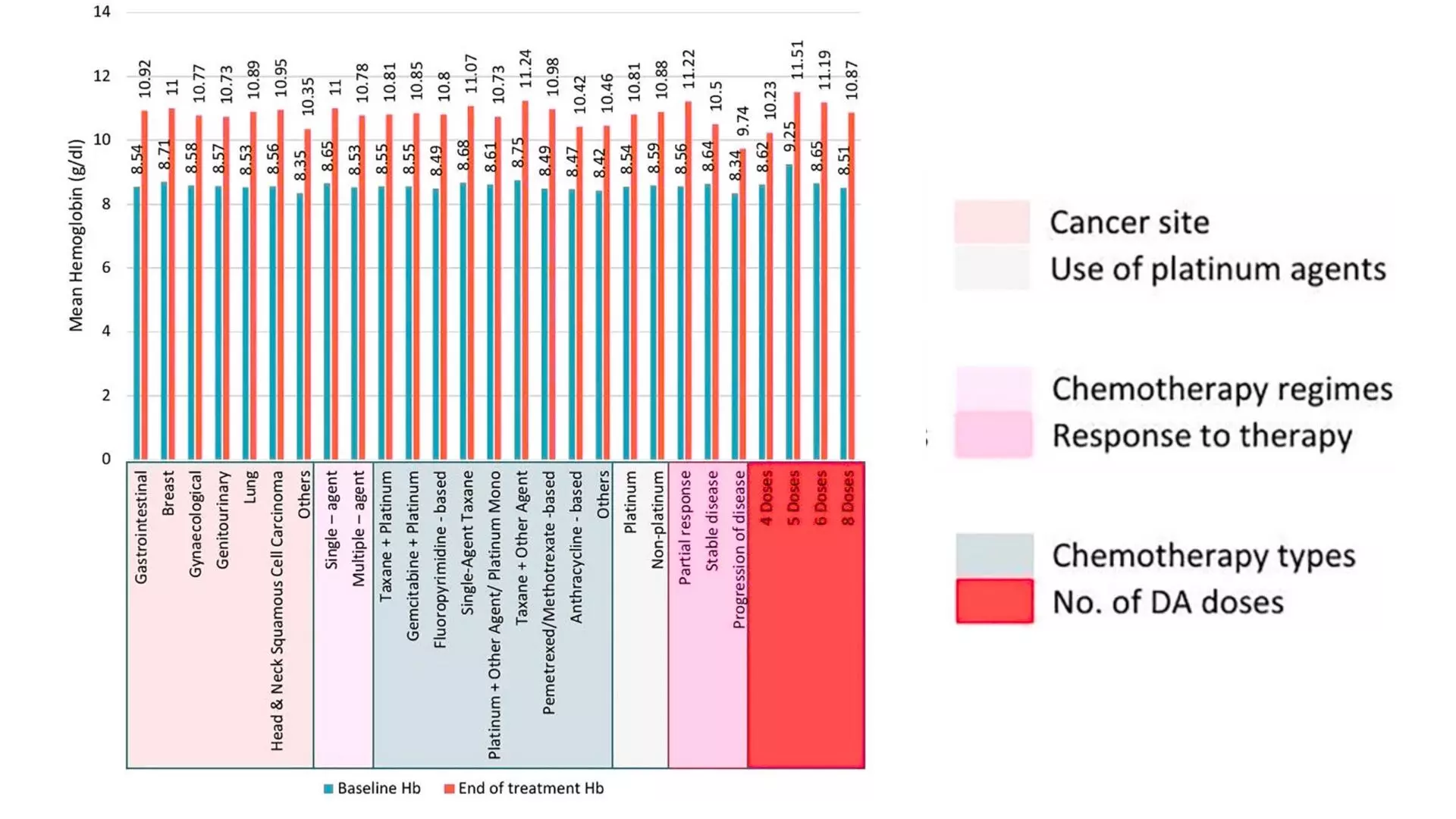
Figure: Mean increment in hemoglobin (Hb) levels across subgroups from baseline to end of treatment. Statistically significant (P<.001) increment in mean Hb levels was recorded across all subgroups.
This study highlighted the efficacy and tolerability of Darbepoetin alfa (Cresp® by Dr Reddy’s Laboratories) in addressing chemotherapy-induced anemia (CIA) in Indian real-world settings. Darbepoetin alfa increased hemoglobin levels, reduced the need for blood transfusions, and improved the quality of life among cancer patients undergoing chemotherapy.
GGI-CO-A1-AQS-300021457-NB-C25-1139
Reference: Chandrakanth M, Agarwala V, Sopory P, Nayak H, Parikh PM, Roy M, De R, Narayan P, Barai AT, Mandal K, Basu M, Kumar S,Uppal RS, Naqvi SMH and Desai R (2025) Cresp®: transforming the landscape of chemotherapy-induced anemia – a comprehensive retrospective real-world analysis in 523 Indian patients.Front. Oncol. 15:1418327.doi: 10.3389/fonc.2025.1418327
Powered by WPeMatico

Kolkata: To improve access to quality healthcare, West Bengal’s first ‘budget hospital’ is set to begin operations inside the SSKM Hospital premises before the upcoming Durga Puja. This innovative project, introduced under the leadership of Chief Minister Mamata Banerjee, marks a new chapter in the state’s healthcare sector.
According to the news reports, the 10-storeyed hospital building, constructed under a Public-Private Partnership (PPP) model, will initially make its first two floors operational for patients. The construction began in November 2023, and the facility promises corporate hospital-like services at a fraction of the cost.
Medical Dialogues had previously reported that Chief Minister Mamata Banerjee has come out with a new concept for Bengal’s health sector, ‘budget hospital’. A plan to set up such a hospital at the SSKM Hospital campus in Kolkata had already been taken.
Also Read: Budget hospitals to be set up across Bengal
The new 10-storey facility will feature a total of 131 beds, comprising 8 VIP suites, 102 tastefully designed cabins, 16 ICU beds, 5 HDU units, and additional recovery beds.
While the treatment pricing is yet to be finalized, patients can expect costs to be 70–80% lower than those in private corporate hospitals.
The project was inspired by the successful PPP model implemented at Woodburn Ward, also located within SSKM, which saw high patient satisfaction and financial viability. Building on that success, the Woodburn Ward is also being upgraded, with the number of cabins being increased from 36 to 60.
According to the Millennium Post, the ‘budget hospitals’ will be set up gradually in all the districts. There are plans to open such a hospital in other medical colleges in Kolkata, such as Calcutta Medical College and Hospital, RG Kar Medical College, NRS Medical College and Hospital, Calcutta National Medical College and Hospital, Burdwan Medical College, North Bengal Medical College, Bankura Sammilani Medical College, etc. These hospitals would be equipped with facilities like private cabins with ACs and TVs, single, double, and four-bedded cabins, ICUs, etc.
Powered by WPeMatico

Popular prescription weight-loss drugs called GLP-1 receptor agonists are now frequently used by type 1 diabetes patients, despite limited data on the drugs’ safety and effectiveness in this patient population, according to a study led by researchers at the Johns Hopkins Bloomberg School of Public Health.
GLP-1 receptor agonists were developed more than two decades ago to treat type 2 diabetes. Some GLP-1 receptor agonists were subsequently approved for reducing cardiovascular disease risk and for treating obesity in the general patient population. Many type 1 diabetes patients began taking GLP-1 receptor agonists-despite the fact that the drugs are thought to put type 1 diabetes patients at risk for developing hypoglycemia, a potentially life-threatening low-blood sugar condition. Because of this risk, patients with type 1 diabetes have been excluded from GLP-1 receptor agonists clinical trials.
About two million Americans have type 1 diabetes, according to the Centers for Disease Control and Prevention. The autoimmune condition occurs in individuals whose bodies do not produce enough insulin and requires lifelong insulin therapy to regulate blood sugar levels.
For their study, the researchers analyzed more than 200,000 de-identified medical records of individuals with type 1 diabetes from 2008 to 2023 and found that the obesity rate among type 1 diabetes patients increased for all age groups and ethnicities during this period.
The analysis also found that the use of GLP-1 receptor agonists rose sharply as the obesity rate increased across groups. In the highest obesity category, the proportion of adult patients using these medications increased from about 4% in 2008–2011 to about 33% in 2020–2023.
The study was published online March 3 in Diabetes, Obesity and Metabolism.
“These findings highlight the urgent need for better data-including clinical trials-on the effectiveness and safety of GLP-1 receptor agonists in people with type 1 diabetes, to inform clear guidelines on their use in these patients,” says study senior author Jung-Im Shin, MD, PhD, an associate professor in the Bloomberg School’s Department of Epidemiology.
The study’s first author, Yunwen Xu, PhD, is a postdoctoral researcher in the same department.
Type 1 diabetes was not treatable until the development of insulin treatments in the 1920s. Historically, people with type 1 diabetes tended to be thin, because insulin deficiency led to the breakdown of fat and muscle for energy, causing weight loss. However, insulin treatments, combined with an environment that promotes weight gain, have contributed to an increased obesity burden in this population and created a demand for GLP-1 receptor agonists.
The new study covered 217,442 patients with type 1 diabetes from more than 30 health systems across the U.S. The data came from electronic health records in a large commercial database and covered the period from October 2008 to September 2023.
The researchers grouped the data by three-year periods, starting with October 2008 to September 2011 and ending with October 2020 to September 2023. In keeping with other recent studies, and with trends in the wider population, they found that the prevalence of obesity among type 1 diabetes patients rose significantly from the first period to the last: from 18% to 26% among youth ages 2–19 and from 30% to 38% among adults age 20 and older. This prominent upward trend was seen among whites, Blacks, Hispanics, and Asians; among men and women; and among patients covered by Medicaid, Medicare, and commercial health insurers.
The rate of GLP-1 receptor agonist use, as measured by prescriptions, rose even faster. This rapid rise occurred in every one of the five weight categories, from normal weight to severe obesity, and among youth and adults. Four percent of severely obese adults with type 1 diabetes had prescriptions for GLP-1 receptor agonists during the 2008–11 period, whereas that figure rose to 33% in 2020–23. Among severely obese youth with type 1 diabetes, fewer than 3% used GLP-1 receptor agonists in 2008–11, but 21%-a sevenfold increase-used them in 2020–23.
As part of the study, the researchers also quantified the use of different GLP-1 receptor agonists in each period.
“In the most recent periods, there were big increases in the use of semaglutide and tirzepatide-the most potent versions of these drugs for weight loss—which again underscores the need for clinical trial data on these patients,” Xu says.
The scientists currently are following up with a more targeted study in type 1 diabetes patients, quantifying the risk of serious hypoglycemia associated with the use of GLP-1 receptor agonists.
Reference:
Yunwen Xu PhD, Justin B. Echouffo Tcheugui MD, Josef Coresh MD, Morgan E. Grams MD, Elizabeth Selvin PhD, Michael Fang PhD, Jung-Im Shin MD, Trends in obesity and glucagon-like peptide-1 receptor agonist prescriptions in type 1 diabetes in the United States, Diabetes Obesity and Metabolism, https://doi.org/10.1111/dom.16300.
Powered by WPeMatico
A new study published in the Journal of American Medical Association showed that younger patients with severe systemic lupus erythematosus (SLE) were more likely to experience a recurrence of pericarditis within the first year following the original diagnosis.
The most prevalent cardiac symptom of systemic lupus erythematosus (SLE) is pericarditis, which is described as inflammation of the serosal sac surrounding the heart. About 20% of SLE patients have pericarditis. From minor chest discomfort that is made worse by resting flat and relieved by leaning forward to crippling symptoms of severe chest pain and dyspnea, patients may have a wide range of symptoms.
The patients may be at higher risk of recurrence due to potential overlapping immunological-mediated pathways, given the widespread immune dysregulation linked to SLE. Yoo Jin Kim and colleagues therefore carried out this study to look at the risk factors and frequency of pericarditis recurrence in SLE patients.
A well-characterized, single-site prospective cohort of a varied group of SLE patients treated at a tertiary medical facility and enrolled between 1988 and 2023 was retrospectively analyzed in this cohort research. The patients with pericarditis who were included in the Hopkins Lupus Cohort were shortlisted.
Pericarditis recurrence was evaluated. Pericarditis was defined using the Safety of Estrogens in Systemic Lupus Erythematosus National Assessment version of the SLE Disease Activity Index (SELENA-SLEDAI). Clinical data from every follow-up visit following the initial pericarditis event was reviewed. Recurrent episodes were those that happened at least six weeks following the initial event that was noted.
In the Hopkins Lupus Cohort, 590 out of 2,931 individuals had a history of pericarditis. Electrocardiograms or specialized imaging were used to confirm the diagnosis of pericarditis in 21 individuals (3.6), with 100% agreement between database and clinical diagnoses. Recurrent pericarditis occurred in 120 patients (20.3%) with a median (IQR) follow-up of 6.7 (2.5-13.6) years and 5,277 years of follow-up. While 59 patients (49.2%) had two or more recurrences, the majority of patients (61 people [50.8%]) only had one recurrence.
Younger age, prednisone therapy for current SLE disease, and duration since the first episode were all significantly linked to recurrence in multivariable analysis. Overall, this study reported the rate of recurrent pericarditis among SLE patients and risk variables linked to recurrence in this cohort analysis of individuals with SLE and a history of pericarditis.
Reference:
Kim, Y. J., Lovell, J., Diab, A., Magder, L. S., Goldman, D., Petri, M., Fava, A., & Adamo, L. (2025). Incidence and factors associated with recurrent pericarditis in lupus. JAMA Network Open, 8(2), e2461610. https://doi.org/10.1001/jamanetworkopen.2024.61610
Powered by WPeMatico
Researchers have found in a new study that in patients with heart failure and iron deficiency, ferric carboxymaltose did not significantly reduce the time to first heart failure hospitalization or cardiovascular death. Further it did not lower the total number of heart failure hospitalizations compared to a placebo, including in patients with a transferrin saturation below 20%.
Uncertainty remains about the efficacy of intravenous iron in patients with heart failure and iron deficiency. A study was done to assess the efficacy and safety of ferric carboxymaltose in patients with heart failure and iron deficiency.
This multicenter, randomized clinical trial enrolled 1105 patients with heart failure (defined as having a left ventricular ejection fraction of ≤45%) and iron deficiency (serum ferritin level <100 ng/mL; or if transferrin saturation was <20%, a serum ferritin level between 100 ng/mL and 299 ng/mL) at 70 clinic sites in 6 European countries from March 2017 to November 2023.
The median follow-up was 16.6 months (IQR, 7.9-29.9 months). Administration of ferric carboxymaltose (n = 558) initially given at an intravenous dose of up to 2000 mg that was followed by 500 mg every 4 months (unless stopping criteria were met) vs a saline placebo (n = 547). The primary end point events were (1) time to cardiovascular death or first heart failure hospitalization, (2) total heart failure hospitalizations, and (3) time to cardiovascular death or first heart failure hospitalization in patients with a transferrin saturation less than 20%. All end point events were measured through follow-up.
The end points would be considered statistically significant if they fulfilled at least 1 of the following conditions: (1) P ≤ .05 for all 3 of the end point comparisons, (2) P ≤ .025 for 2 of the end point comparisons, or (3) P ≤ .0167 for any of the 3 end point comparisons (Hochberg procedure).
Results Of the 1105 participants (mean age, 70 years [SD, 12 years]; 33% were women), cardiovascular death or first heart failure hospitalization (first primary outcome) occurred in 141 in the ferric carboxymaltose group vs 166 in the placebo group (hazard ratio, 0.79 [95% CI, 0.63-0.99]; P = .04). The second primary outcome (total heart failure hospitalizations) occurred 264 times in the ferric carboxymaltose group vs 320 times in the placebo group (rate ratio, 0.80 [95% CI, 0.60-1.06]; P = .12). The third primary outcome (cardiovascular death or first heart failure hospitalization in patients with a transferrin saturation <20%) occurred in 103 patients in the ferric carboxymaltose group vs 128 patients in the placebo group (hazard ratio, 0.79 [95% CI, 0.61-1.02], P = .07).
A similar amount of patients had at least 1 serious adverse event in the ferric carboxymaltose group (269; 48.2%) vs in the placebo group (273; 49.9%) (P = .61). In patients with heart failure and iron deficiency, ferric carboxymaltose did not significantly reduce the time to first heart failure hospitalization or cardiovascular death in the overall cohort or in patients with a transferrin saturation less than 20%, or reduce the total number of heart failure hospitalizations vs placebo.
Reference:
Anker SD, Friede T, Butler J, et al. Intravenous Ferric Carboxymaltose in Heart Failure With Iron Deficiency: The FAIR-HF2 DZHK05 Randomized Clinical Trial. JAMA. Published online March 30, 2025. doi:10.1001/jama.2025.3833
Powered by WPeMatico

A global study by experts at the University of Sydney has shown that countries which consume more plant-based proteins-such as chickpeas, tofu and peas-have longer adult life expectancies.
Published in Nature Communications, Dr Alistair Senior, PhD candidate Caitlin Andrews and their team in the Charles Perkins Centre studied food supply and demographic data between 1961-2018 from 101 countries, with the data corrected to account for population size and wealth, to understand whether the type of protein a population consumed had an impact on longevity.
First author Caitlin Andrews said: “Our study suggests a mixed picture when it comes to comparing the health impacts of meat- versus plant-based protein at a population level.
“For the under-fives, a food system that supplies large amounts of animal-based proteins and fats – such as meat, eggs and dairy – lowered rates of infant mortality. However, for adults, the reverse was true, where plant-based proteins increased overall life expectancy.”
To understand the impact of plant- and animal-based protein diets on human longevity, the researchers analysed publicly available data about the food supply of 101 countries across a 60-year period. The data included the amount of food produced per country, along with the levels of calories, proteins and fats available for consumption.
The countries studied represented a range of food systems, including countries where the consumption of animal-based protein is higher, such as Australia, the US, Sweden and Argentina, and areas where the consumption of plant-based foods is more prevalent such as Pakistan and Indonesia.
In order to compare the impact of different countries’ food supplies on life expectancy, the researchers corrected the data to take into account the differences in wealth and population size between countries. Having done this, they found that countries where overall availability of plant-based proteins were higher, such as India, had relatively longer life expectancies than countries where animal-based proteins was more readily available, such as the US.
Eating high levels of animal-based protein, particularly processed meat, has long been linked to a range of chronic conditions such as cardiovascular disease, type 2 diabetes and certain types of cancer.
Meanwhile, plant proteins – including legumes, nuts and whole grains – are associated with a lower risk or chronic diseases and overall mortality rates, with studies suggesting that plant-based diets have contributed to the longevity in the most long-lived communities on the planet – Okinawa in Japan, Ikaria in Greece and Loma Linda in California.
Lead investigator Dr Senior said: “Protein is a crucial part of the human diet, but as eating habits change and developed countries look to decarbonise, where we get our protein from has come under greater scrutiny.
“The knowledge that plant-based protein is associated with a longer life is really important as we consider not only how our diets impact our own longevity, but the health of the planet.”
Reference:
Andrews, C.J., Raubenheimer, D., Simpson, S.J. et al. Associations between national plant-based vs animal-based protein supplies and age-specific mortality in human populations. Nat Commun 16, 3431 (2025). https://doi.org/10.1038/s41467-025-58475-1
Powered by WPeMatico
