Enhancing Adherence to Perioperative Antibiotic Prophylaxis Guidelines: Key Step for Prevention of Surgical Site Infections
Recent qualitative study aimed to explore factors influencing perioperative antibiotic administration and assess the potential impact of a clinical decision support tool on improving adherence to Infectious Disease Society of America (IDSA) guidelines for surgical antibiotic prophylaxis. The researchers conducted semistructured interviews with a diverse group of perioperative personnel, including anesthesiologists, surgeons, nurses, residents, and pharmacists across the Yale-New Haven Health System. After 3 pilot interviews, 9 sessions were conducted with a total of 17 participants. Several key themes emerged from the interviews: 1. Limited familiarity with IDSA antibiotic guidelines – There was significant uncertainty regarding the existence and content of IDSA guidelines, with practitioners often relying on Surgical Care Improvement Project (SCIP) guidance instead. 2. Lack of standardization and optimization of the antibiotic decision-making process – While electronic health record resources and pharmacy consultation were available, access and usability were variable, leading to inconsistent workflows. 3. Challenges with managing beta-lactam allergies and optimizing vancomycin timing – Practitioners expressed uncertainty about appropriate antibiotic choices for patients with allergies and difficulty ensuring optimal timing of vancomycin administration. 4. Perceived benefit of a clinical decision support tool – Interviewees felt that a well-designed tool integrated into the electronic health record could enhance workflow and promote better adherence to perioperative antibiotic guidelines. The interviews provided important insights into the barriers to guideline adherence and potential opportunities for improvement through the development of a tailored clinical decision support tool. Key recommendations included improved visibility and accessibility of guidelines, better integration of pharmacy input, and more reliable processes for managing antibiotic administration, especially for challenging scenarios like vancomycin timing and antibiotic allergies.
Key Points
1. The study aimed to explore factors influencing perioperative antibiotic administration and assess the potential impact of a clinical decision support tool on improving adherence to Infectious Disease Society of America (IDSA) guidelines for surgical antibiotic prophylaxis.
2. The researchers conducted semistructured interviews with a diverse group of perioperative personnel, including anesthesiologists, surgeons, nurses, residents, and pharmacists across the Yale-New Haven Health System, and identified several key themes: – Limited familiarity with IDSA antibiotic guidelines, with practitioners often relying on Surgical Care Improvement Project (SCIP) guidance instead. – Lack of standardization and optimization of the antibiotic decision-making process, with variable access and usability of electronic health record resources and pharmacy consultation. – Challenges with managing beta-lactam allergies and optimizing vancomycin timing, leading to uncertainty about appropriate antibiotic choices and difficulty ensuring optimal timing of vancomycin administration. – Perceived benefit of a well-designed clinical decision support tool integrated into the electronic health record, which could enhance workflow and promote better adherence to perioperative antibiotic guidelines.
3. The interviews provided insights into the barriers to guideline adherence and potential opportunities for improvement, including improved visibility and accessibility of guidelines, better integration of pharmacy input, and more reliable processes for managing antibiotic administration, especially for challenging scenarios like vancomycin timing and antibiotic allergies.
4. The study highlighted the need for a tailored clinical decision support tool to address the identified barriers and improve adherence to perioperative antibiotic guidelines.
5. The findings suggest that a well-designed clinical decision support tool integrated into the electronic health record could enhance workflow and promote better adherence to IDSA guidelines for surgical antibiotic prophylaxis.
6. The study emphasizes the importance of addressing the identified barriers, such as limited familiarity with guidelines, lack of standardization in the antibiotic decision-making process, and challenges with managing complex scenarios, in order to improve adherence to perioperative antibiotic guidelines.
Reference –
Amit Bardia et al. (2024). Individual And System Level Factors Contributing To Guideline Non-Adherent Surgical Antibiotic Prophylaxis At A Tertiary Health Care System: A Qualitative Analysis.. *Anesthesiology*. https://doi.org/10.1097/ALN.0000000000005302.
Powered by WPeMatico






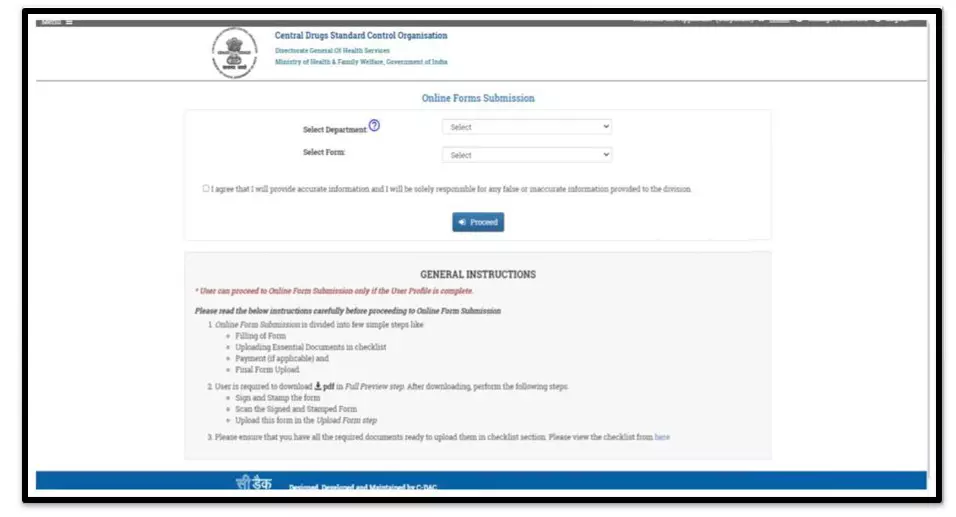
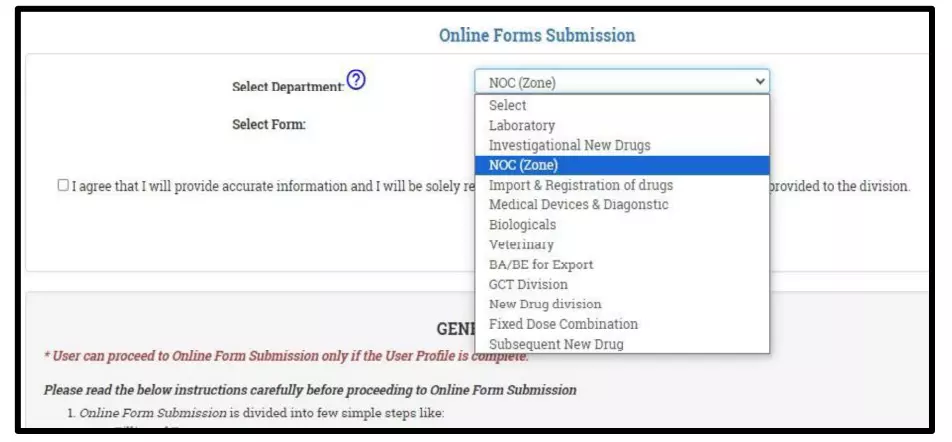
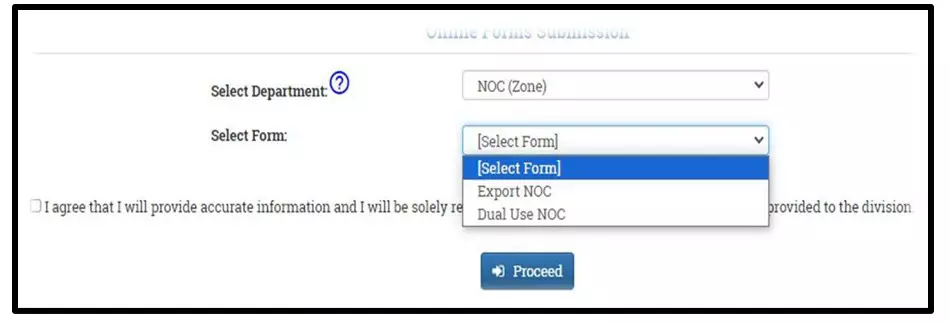
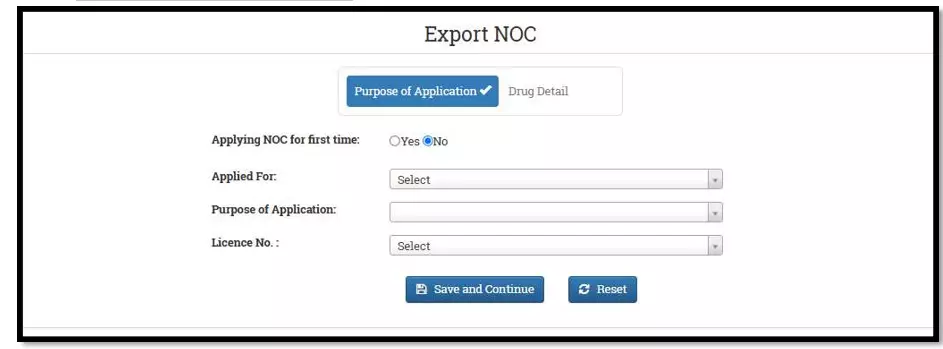
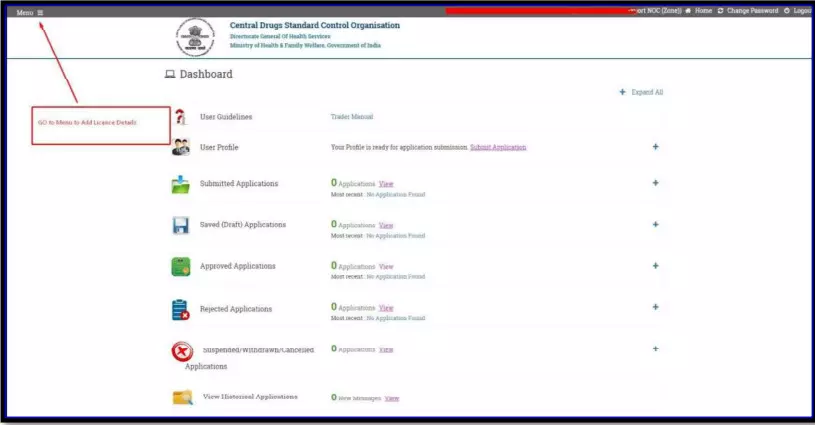
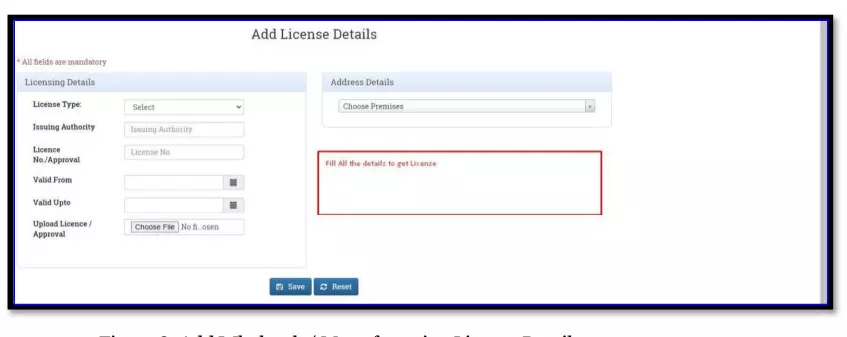
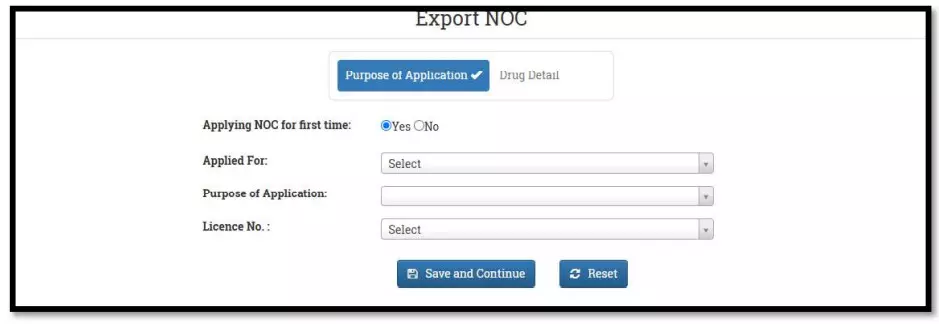
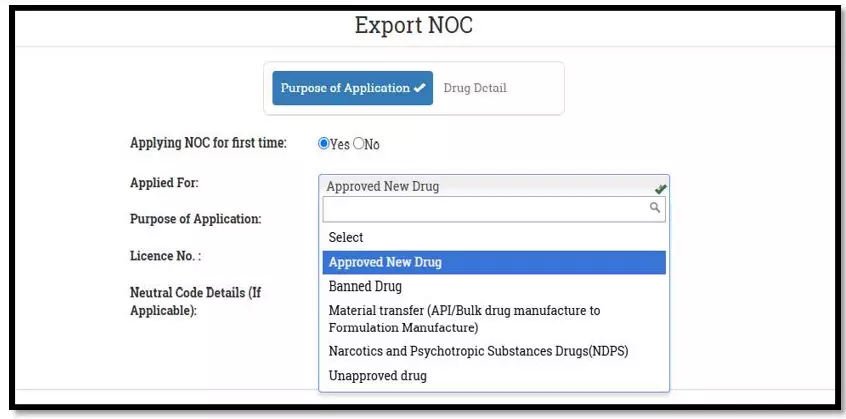
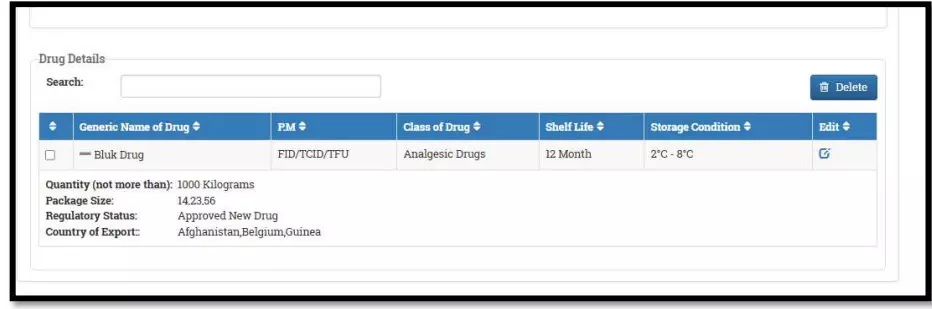
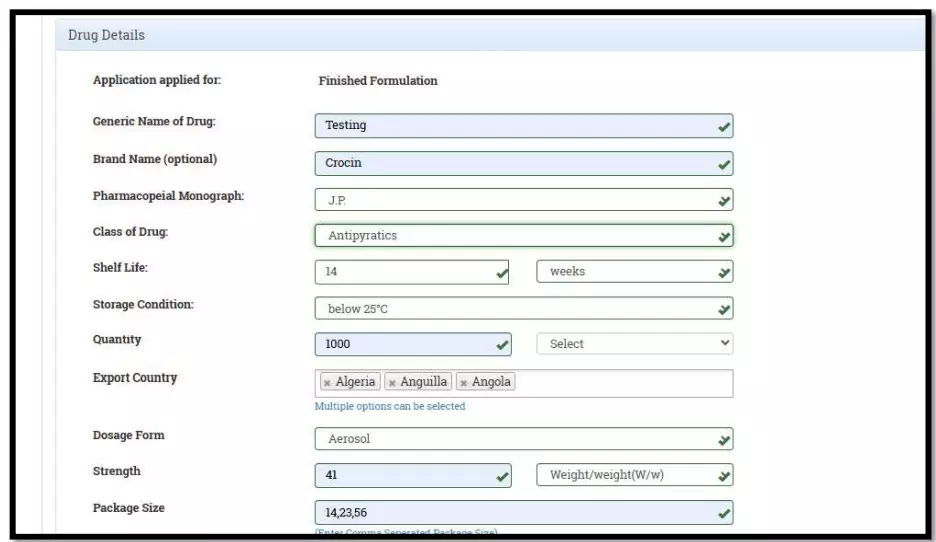
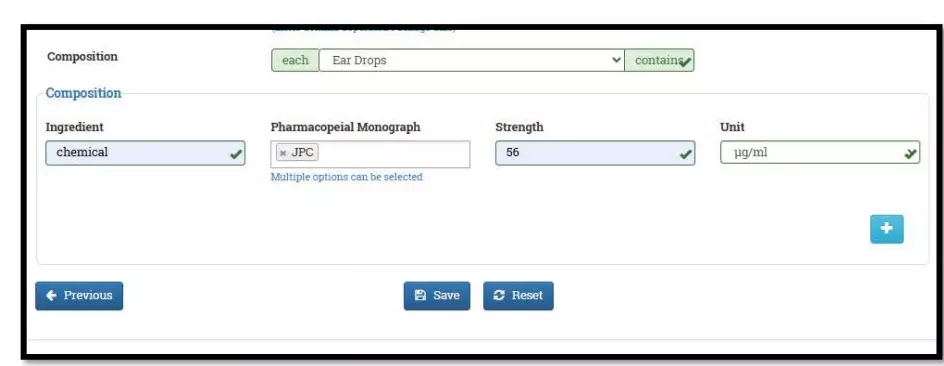
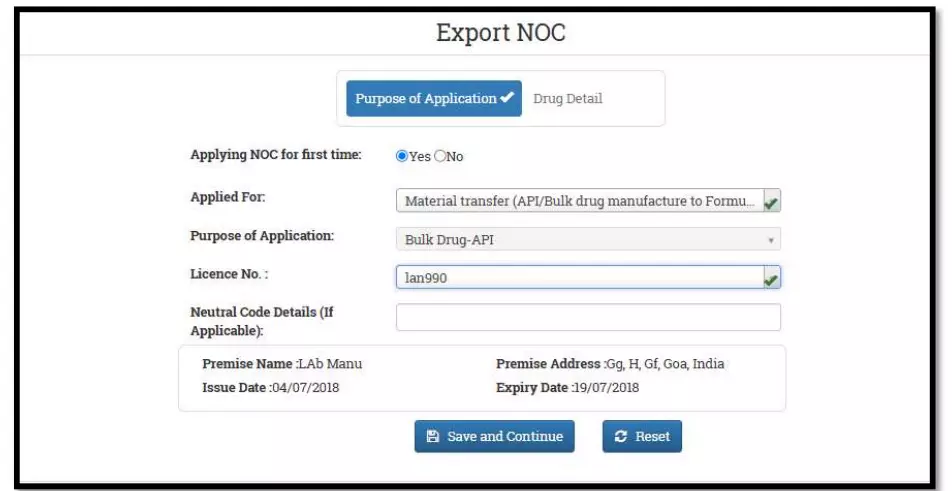
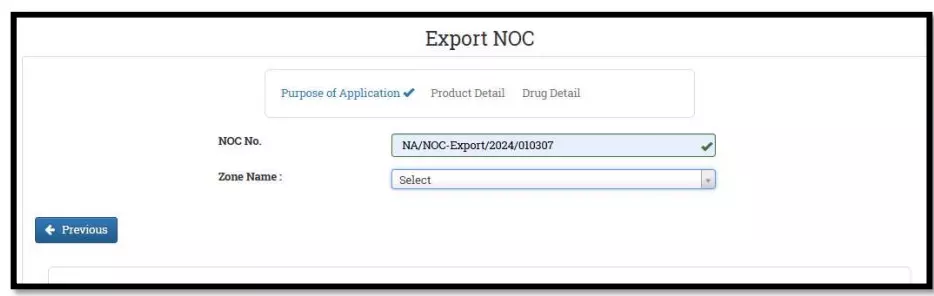
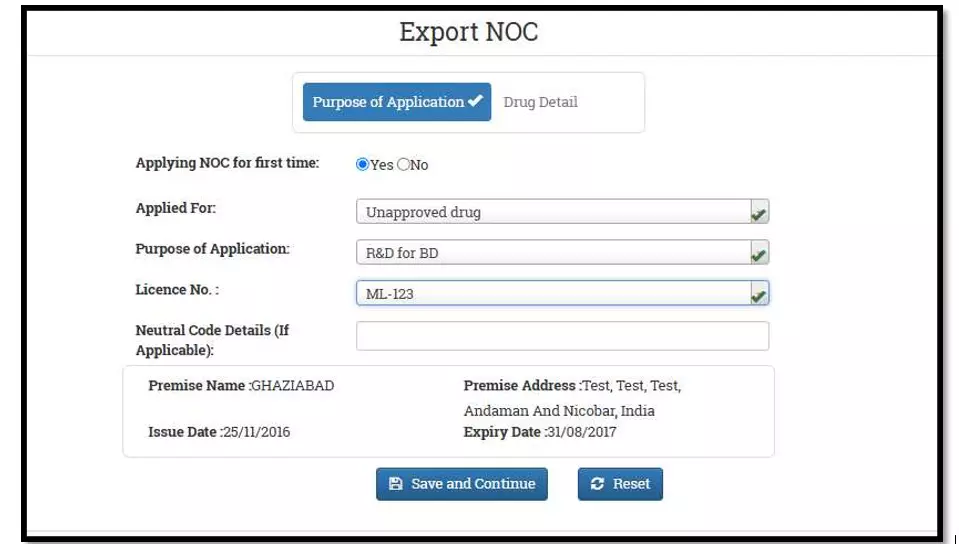
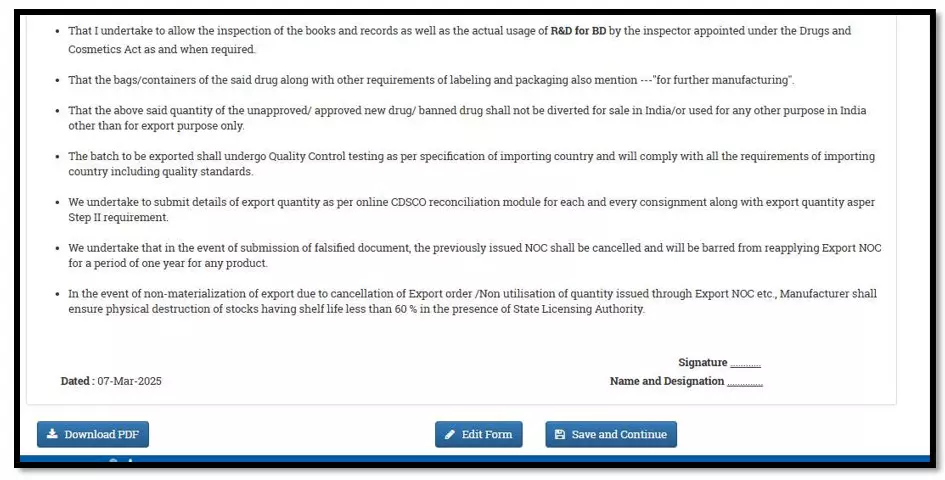
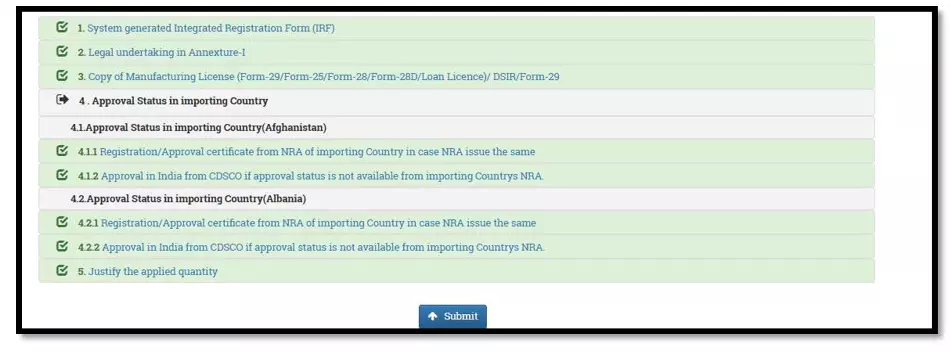
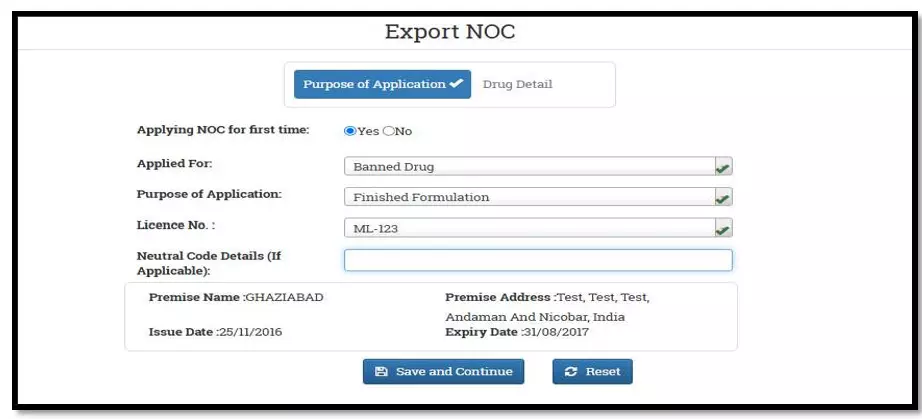 After selecting the Finished Formulation option and Licence No (How to manufacturing license number please referee page No. 6) from the drop-down user have to click on save and continue button for drug details entry as attached screenshots.
After selecting the Finished Formulation option and Licence No (How to manufacturing license number please referee page No. 6) from the drop-down user have to click on save and continue button for drug details entry as attached screenshots.