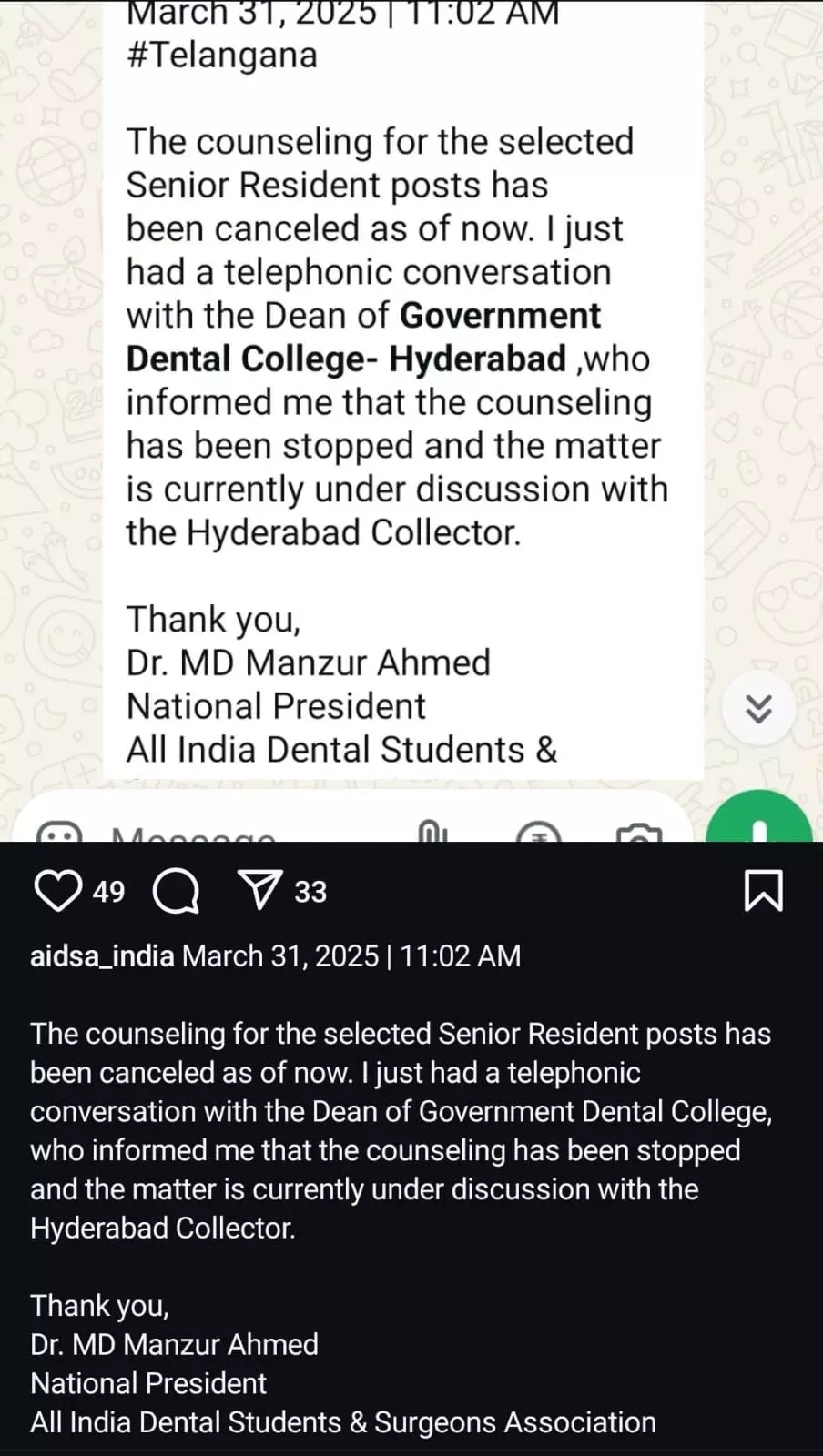
New IVF method mimics fallopian tube environment, increasing sperm viability ,reveals research
The success of in vitro fertilization depends on many factors, one of which is sperm viability. A recent study from the University of Illinois Urbana-Champaign documents a new way to select viable sperm and prolong their viability in the laboratory, reducing one source of variability during the process.
“The fallopian tube in women, or the oviduct, has an ability to lengthen sperm lifespan that, until now, we couldn’t recreate in IVF. In 2020, we discovered that complex sugars called glycans are the components of the oviduct that can bind and store sperm and keep them alive,” said senior study author David Miller, professor in the Department of Animal Sciences, part of the College of Agricultural, Consumer and Environmental Sciences at Illinois.
Miller’s group collaborated with chemists to test hundreds of oviduct glycans for their ability to bind pig sperm, settling on one called sulfated Lewis X trisaccharide, or suLeX, for further testing. They focused on pig sperm not only as a proof of concept for future human studies, but also because animal agriculture relies on IVF, too. In pig IVF, multiple sperm often fertilize single eggs, resulting in inviable embryos. The hope with using glycans was that fewer free-swimming sperm would approach and fertilize eggs simultaneously.
The researchers attached suLeX to the bottom of culture dishes, then added sperm. The sperm were given 30 minutes to adhere to the compounds before the researchers began adding eggs, introducing them 0, 6, 12, or 24 hours later.
“By adding eggs at later time points, we could test the system to see whether suLeX increased the longevity of the sperm. Essentially, we found we can maintain or extend fertilization rates over time, increasing the window of successful IVF,” Miller said.
At 0 hours, IVF efficiency (fertilized zygotes vs. total number of eggs) was significantly greater with sperm that were initially attached to suLeX (at 53%) than a control with no oviduct compounds (36%) and two alternative “control” compounds (about 40% each).
The time delays decreased fertilization rates for all groups, but less so for suLeX. In the control group with no oviduct glycans, fertilization was down to 1% at the 24-hour time point. But with suLeX, 12% of the eggs were fertilized after 24 hours.
The IVF setup with suLeX droplets also allowed the researchers to wash away free-swimming sperm before introducing eggs.
“Because the sperm were bound securely to the glycan compound, we could reduce the overall number of sperm, which meant fewer cases where more than one sperm fertilized the eggs,” Miller said.
The foundational study could one day improve IVF success for both animals and humans.
“There are companies, especially related to dairy cattle, that use IVF to produce and sell high-genetic-merit embryos that, after they are delivered, will produce milk more efficiently,” Miller said. “This technology could potentially help produce meat and milk more efficiently.”
He added that the specific glycans that bind human sperm have not yet been identified, but once that happens, glycan-IVF could help with timing mismatches between egg maturity at harvest and sperm viability in humans.
“Both eggs and sperm have to undergo a maturation phase before they’re ready for fertilization, so the timing is critical. There’s variability in the time it takes sperm to complete their final major maturation step,” Miller said. “We think glycan-IVF could lengthen the fertile window of sperm and possibly increase IVF rates, though we need further testing to verify that.”
Reference:
Soto-Heras, S., Volz, L.J., Bovin, N. et al. Porcine sperm bind to an oviduct glycan coupled to glass surfaces as a model of sperm interaction with the oviduct. Sci Rep 15, 4680 (2025). https://doi.org/10.1038/s41598-025-88986-2
Powered by WPeMatico