IPC issues Safety warning against use of common painkiller Meftal
India: The Indian Pharmacopoeia Commission (IPC) has recently issued a drug safety alert on the common painkiller Meftal. The IPC is an autonomous institution of the Ministry of Health that sets standards for all drugs that are manufactured, sold, and consumed in India.
The IPC has advised consumers and healthcare professionals (HCPs) to exercise caution concerning the potential adverse drug reaction (ADR) linked to the use of Mefenamic acid, the constituent of Meftal.
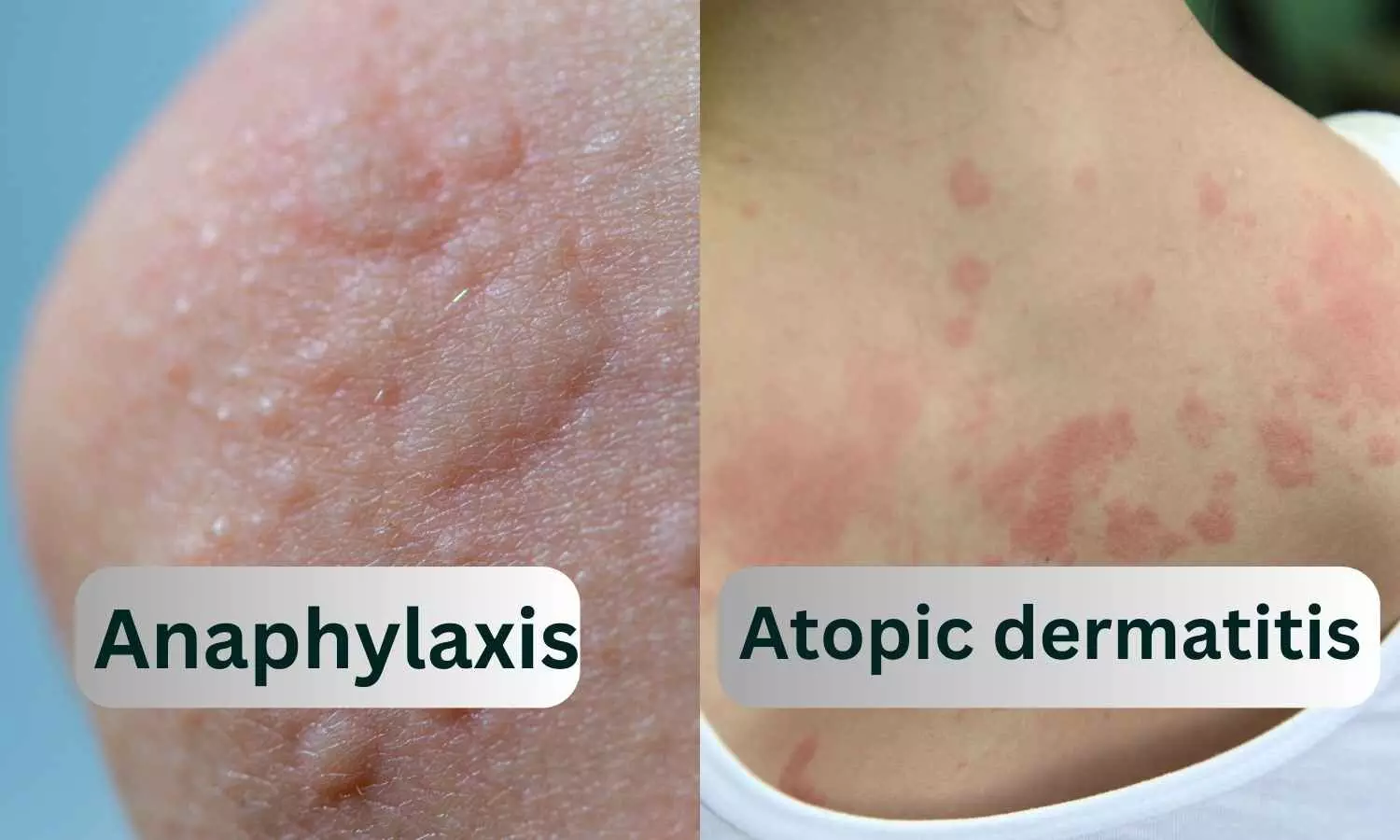
A preliminary analysis of ADRs from the database of the Pharmacovigilance Programme of India has revealed cases of Drug Rash with Eosinophilia and Systemic Symptoms (DRESS) syndrome, which affects internal organs. These symptoms may appear 2-8 weeks after taking the drug.
“The IPC has advised healthcare professionals, patients/consumers to closely monitor the possibility of the above adverse drug reaction associated with the use of the suspected drug,” stated the alert, issued on 30 November.
The alert further stated, “If such a reaction is encountered, people should report the matter to the national coordination centre of the PvPI under the commission by filling a form on the website — www.ipc.gov.in — or through the Android mobile app ADR PvPI, as well as PvPI helpline number 1800-180-3024.”
DRESS syndrome, also known as Drug-Induced Hypersensitivity Syndrome (DIHS), is a severe allergic reaction affecting around 10% of individuals, potentially deadly and caused by certain medications. It is characterized by high fever, skin rash, swollen lymph nodes and complications in internal organs.
The key features of DRESS syndrome are:
· Skin Rash: Patients with DRESS often develop a widespread rash that can involve various body parts. The rash may be maculopapular (flat and raised lesions), erythematous (red), and may resemble other skin conditions.
· Internal Organ Involvement: DRESS can affect multiple organs, including the liver, kidneys, lungs, and heart. This can lead to hepatitis, nephritis, pneumonitis, and myocarditis.
· Fever: Individuals with DRESS typically experience a high fever.
· Lymphadenopathy: Swollen lymph nodes (lymphadenopathy) are common in DRESS.
· Haematologic Abnormalities: Blood abnormalities, such as atypical lymphocytes, eosinophilia (elevated eosinophil count), and other hematologic changes, may be present.
Use and Associated Side Effects of Meftal
Meftal is used commonly as a non-steroidal anti-inflammatory drug (NSAID). It is indicated for the treatment of osteoarthritis, rheumatoid arthritis, dysmenorrhoea, mild to moderate pain, fever, inflammation, and dental pain.
Although the drug is not available over the counter (OTC) and requires a prescription for purchase, it is extensively used in India for various purposes such as relieving headaches, menstrual pain, and muscle and joint pain, and is even prevalent among children for high fever.
Prolonged use of Meftal is reported to increase the risk of stomach ulcers, bleeding, and related complications. It has been associated with potential adverse effects on the cardiovascular system. Some experts have flagged renal complications as a potential side effect of Meftal.
If someone suspects DRESS or experiences symptoms after taking a medication, it is important to seek prompt medical attention. DRESS is a medical emergency, and early recognition and intervention are required for a better prognosis. Consultation with healthcare professionals is recommended for accurate diagnosis and appropriate management.
Powered by WPeMatico