Widespread Dental Pulp Calcification Observed in SLE Patient on Long-Term Steroid Therapy: Case Report
China: A recent case study published in The Open Dentistry Journal sheds light on a dental issue faced by patients with systemic lupus erythematosus (SLE) treated with long-term glucocorticoids (GCs). SLE is an autoimmune disease that causes widespread inflammation, and glucocorticoids are often prescribed to manage its symptoms. However, prolonged use of these medications can lead to complications, including pulp cavity calcification in the teeth.
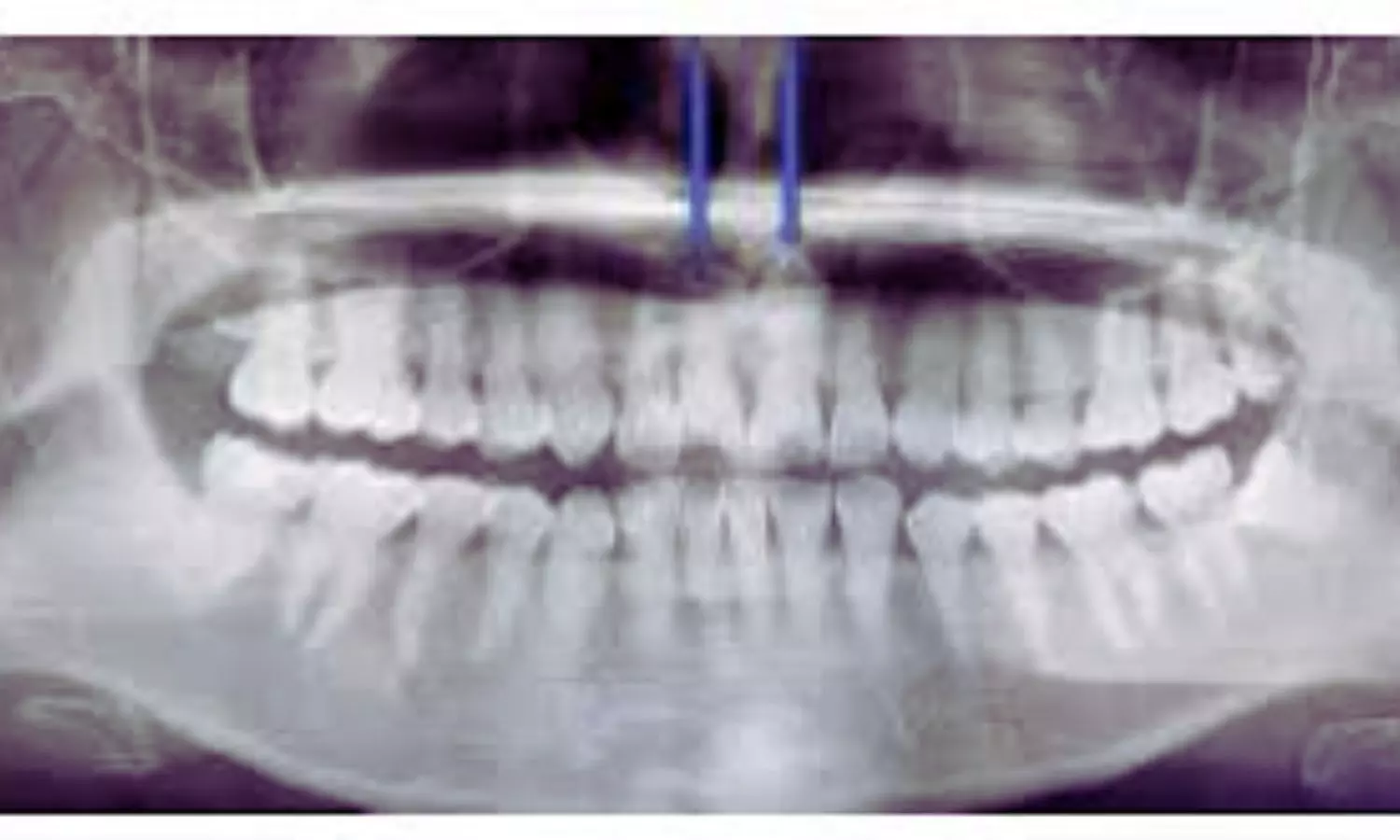
The case involved a 30-year-old woman diagnosed with SLE at 17 and treated with methylprednisolone for 13 years. She visited the dental clinic with a fractured tooth (tooth 11) and reported sensitivity to cold. Examination and diagnostic tests, including electric pulp testing and panoramic radiographs, revealed chronic pulpitis and calcification of the pulp cavity in the affected teeth. Cone beam computed tomography (CBCT) confirmed that pulp calcification extended beyond the damaged teeth.
The long-term use of glucocorticoids raised concerns about the role these medications might play in the calcification of her dental pulp. While electric pulp testing indicated normal pulp sensitivity, the radiographs and CBCT scans revealed root canal calcification, complicating the treatment. Initially, a root canal treatment (RCT) was recommended, but the calcification made it difficult to access the root apex.
Given the severity of calcification, the treatment plan was modified. A pulpotomy was performed, and the root canals were temporarily sealed with zinc oxide-eugenol cement. Follow-up imaging confirmed that calcification affected all of the patient’s teeth, making traditional RCT less viable. Further treatment included calcium hydroxide and composite resin to seal the canals, followed by dental crown preparation.
After a year of follow-up, the patient reported no discomfort or inflammation. However, the calcification in the teeth remained a concern. This case highlights the challenges of treating dental issues in patients with SLE on long-term glucocorticoid therapy. The study emphasizes the need for regular dental check-ups for these patients and suggests that a multidisciplinary approach is necessary to address their medical and dental needs.
The case study also emphasizes the importance of early intervention in preventing long-term dental complications in SLE patients. As glucocorticoids can cause significant dental issues, healthcare providers should work together to ensure comprehensive care and minimize the impact of such treatments on oral health.
“Based on the patient’s long-term glucocorticoid use and supporting studies, it appears that extended GC therapy significantly contributes to widespread dental pulp calcification,” Shenjie Xu, Department of Oral Medicine, The Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, Zhejiang, P.R. China, and colleagues noted. They added, “When multiple teeth are involved, root canal treatment becomes unfeasible.”
They concluded, “Hence, a multidisciplinary approach is essential for managing SLE or other chronic conditions in patients on prolonged GC therapy.”
Reference:
DOI: 10.2174/0118742106321913240820051043
Powered by WPeMatico