Organoid model integrates microglia to study inflammation in brain
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico

Pune: Pune’s maternity centers are facing a growing crisis despite ongoing efforts to address critical gaps in their services. A 2023 gap analysis by the Pune Municipal Corporation (PMC) revealed several critical issues, such as a shortage of specialist doctors, the absence of blood banks, a lack of ICU units, and an inability to manage complicated cases.
Despite efforts to tackle these challenges, the shortage of qualified medical staff continues to be a pressing concern.
Medical Dialogues had earlier reported that in response to a pressing shortage of healthcare experts in the Pune Municipal Corporation (PMC) run healthcare facilities, three ward medical officers (WMO) were transferred to maternity homes on Saturday. The Pune Municipal Corporation (PMC) has been suffering from a shortage of expert doctors and staff. This lack of specialized doctors eventually caused inconvenience to citizens and patients.
Also Read: Pune Municipal Corporation transfers 3 gynaecologists to maternity homes
According to the news reports, PMC operates 21 maternity centres across the city, but a recent investigation has uncovered troubling gaps in the infrastructure and staffing. Of these 21 centres, two do not have operating theatres, which are essential for performing C-sections in case of emergencies. The remaining 19 centres, which do have operating theatres, are managed by just eight gynecologists.
In some cases, women in need of a C-section are rushed to hospitals that have the necessary medical staff, often at the last minute, which can delay crucial medical procedures.
These gynecologists are primarily stationed at Kamala Nehru Hospital, Sonawane Hospital, and Rajiv Gandhi Hospital, where the majority of deliveries take place. Despite the inauguration of a medical college alongside Kamala Nehru Hospital in 2022, the situation remains dire. Dr. Lata Trimbake, the medical superintendent of all the maternity centres, stated that despite having the college, resident doctors, and more manpower have not been secured.
The staff shortage has reached critical levels, with only six anesthetists available to cover all 19 maternity centers. This limited number means that only a few facilities are capable of performing C-sections or providing the level of care needed for high-risk pregnancies.
Also Read: Pune Municipal Corporation issues notice to 24 Private Hospitals
The shortage of skilled medical staff and the lack of proper infrastructure pose serious risks to pregnant women, especially those with complicated pregnancies. Officials mentioned that patients admitted to smaller centers are often instructed to either go directly to Kamala Nehru Hospital or are referred to Sassoon Hospital.
Powered by WPeMatico

Agartala: To ease the burden on underprivileged patients and their families, the Agartala Government Medical College (AGMC) and Govind Ballabh Pant (GBP) Hospital have launched a midday meal service priced at just Rs 10.
The initiative was launched in collaboration with the Rotary Club and the Patient Welfare Committee and aims to ease the financial burden on patients. Many of them travel from outside Agartala to GBP Hospital, one of the region’s oldest medical institutions, for treatment, news agency ANI reported.
Recognising the financial strain on these patients and their families, the hospital administration, along with the state government, has ensured that wholesome meals, including eggs, vegetables, and lentils, are made available at a nominal cost with support from the state government.
Speaking to ANI during the launch, the Tripura CM said, “Agartala Government Medical College and Govind Ballabh Pant Hospital, commonly known as GBP Hospital, is one of the oldest hospitals. It currently has 150 undergraduate students and about 89 postgraduate students.
Many patients come here from outside the city… I have worked at this hospital before, and recently, discussions were ongoing about the fact that patients often have family members accompanying them. In collaboration with the Rotary Club and the Patient Welfare Committee, a midday meal service has now been launched, providing meals for just Rs 10.
I personally provided a meal to a patient, and they were very happy. You can understand how significant it is to get a meal for Rs 10, which includes eggs, vegetables, and lentils.”
In addition to the meal service, a new shelter facility has also been constructed for families accompanying patients. This initiative ensures they have a place to stay and access to affordable food, significantly reducing their hardships.
“As the Honourable Prime Minister has emphasised the importance of such initiatives, we have ensured that this programme remains within our budget. Additionally, we are constructing a shelter room for those coming from outside, where they can stay and have meals. This facility has already been launched, and we are working further to enhance it,” Saha added.
One of the meal service beneficiaries, Pramila Debnath, who was also a family member of patients visiting the hospital, expressed her gratitude to the Chief Minister and said that this scheme would greatly benefit the poor.
“The Honourable Chief Minister recently introduced an initiative where a midday meal is available for just 10 rupees. I am very happy about this, as it has greatly benefitted the poor. This kind of facility was never available before, and I truly appreciate it,” Debnath said.
Another beneficiary of the meal service, Subhash Baishnab, said, “I really liked this initiative. For the past one and a half months, we have been here, but such a facility was not available before. Previously, we had to eat outside, whether it was day or night. But today, we have received this opportunity from the Tripura Government, and I truly appreciate it. I would like to tell the Chief Minister that no matter how many generations come in the future, we always hope to have a government like this that cares for us.”
With this meal service, the state government remains committed to further enhancing the programme, ensuring that every patient and their family receive adequate support during their stay at GB Hospital.
Powered by WPeMatico

Mumbai: The much-anticipated
Maharashtra Medical Council (MMC) elections are scheduled for Thursday, April
3. A total of approximately 1.30 lakh registered doctors across the state will cast their votes to elect nine members who will serve on the council and oversee the regulation of the medical profession for the next five years.
The elections will be conducted via a secret ballot at designated polling centres in each district, under the supervision of the district collector. In Nagpur, voting will be held at Hostel No. 3, situated near the dean’s office at Government Medical College (GMC). The city will feature 16 polling booths, catering to the district’s 7,670 registered medical professionals, including 3,941 male and 3,729 female doctors. Polling stations will be open from 8 a.m. to 5 p.m., allowing ample time for medical practitioners to participate in the election process.
The Maharashtra Medical
Council serves as a quasi-governmental and quasi-judicial body responsible for
regulating medical practitioners across the state. With over two lakh
registered doctors under its purview, the Maharashtra Medical Council plays a pivotal role in
maintaining ethical medical standards, granting licences, investigating
complaints, and ensuring scientific advancements in medical practice, reports the Times of India. The
elected members will be responsible for making crucial decisions that impact
both healthcare professionals and patients throughout Maharashtra.
Currently, the MMC is
functioning under an administrator, as the term of the previously elected
general body concluded during the COVID-19 pandemic, delaying the elections.
Before the pandemic, the council was led by a body primarily composed of Indian
Medical Association (IMA) members. The IMA has once again introduced a panel of
nine candidates for this election, while the Parivartan panel is also
competing. Additionally, several independent candidates and other groups have
entered the contest, making this a highly competitive election, reports the Daily.
With 38 polling centres
set up across Maharashtra, the elections will play a crucial role in
determining the future course of medical regulations in the state. The nine
elected members will serve a five-year term, taking charge of vital
responsibilities that will shape the medical profession in Maharashtra. The
collector’s office said, “Only registered medical
practitioners with names on the electoral roll are eligible to vote.
Voters must carry one valid photo ID for verification.”
Medical
Dialogues had earlier reported that a medical practitioner from New Panvel has filed a
petition in the Bombay
High Court challenging the state medical education department, the
MMC, and returning officer Shilpa Parab on various grounds, including the
preparation of the electoral list and the returning officer’s eligibility.
Meanwhile Bombay High
Court refused to interfere with the upcoming MMC election scheduled for April 3.
The court was hearing a petition filed by Dr. Sudhir Naik and seven other
doctors, who challenged the election over concerns of low voter turnout. The
petition highlighted that the polling is scheduled for a weekday and has
limited centres. The court directed the MMC to increase the number of booths in
future elections to ensure better voter participation.
Powered by WPeMatico

USA: The STRIDE trial has demonstrated that semaglutide significantly enhances walking capacity in individuals with peripheral artery disease (PAD) and type 2 diabetes (T2D). After 52 weeks of treatment, patients receiving semaglutide experienced a 21% improvement in maximum walking distance compared to just 8% in the placebo group.
“The study found a significant difference between the two groups, with an estimated treatment ratio of 1.13 (95% CI 1.06–1.21; p=0.0004), highlighting the potential of semaglutide in improving mobility and quality of life for patients with PAD and diabetes,” Marc Bonaca, of CPC Clinical Research and University of Colorado School of Medicine in Aurora reported at the American College of Cardiology (ACC) annual meeting. The study was also published online in The Lancet.
PAD, a progressive vascular disease that restricts blood flow to the lower limbs, affects over 230 million people worldwide and is associated with reduced functional capacity and increased cardiovascular risk. Despite its widespread prevalence, effective treatment options to enhance mobility and overall well-being remain limited. In the STRIDE trial, a phase 3b, double-blind, randomized, placebo-controlled study, the researchers aimed to assess whether semaglutide could improve walking ability and alleviate PAD-related symptoms in patients with concurrent diabetes.
Conducted across 112 clinical sites in 20 countries, the trial enrolled 792 participants aged 18 years and older, all diagnosed with T2D and PAD with intermittent claudication. Eligible participants were required to have a Fontaine stage IIa classification (walking ability over 200 meters) and an ankle-brachial index of 0.90 or lower or a toe–brachial index of 0.70 or lower. Participants were randomly assigned in a 1:1 ratio to receive either a weekly 1.0 mg subcutaneous injection of semaglutide or a placebo for one year.
The study results showed that patients receiving semaglutide demonstrated superior improvements in walking ability compared to those given a placebo. In absolute terms, semaglutide led to a median improvement of 26.4 meters over the placebo in maximum walking distance, suggesting its effectiveness in addressing the mobility limitations associated with PAD in diabetic patients.
In terms of safety, the incidence of treatment-related adverse events was low. Serious adverse events were reported in 1% of patients in the semaglutide group and 2% in the placebo group, with gastrointestinal issues being the most common. Importantly, no treatment-related deaths were observed.
“These findings reinforce the potential of semaglutide as a therapeutic option for improving functional outcomes in patients with PAD and T2D. Further research is warranted to explore its benefits in PAD patients without diabetes and to better understand the mechanisms underlying its positive effects on vascular health and mobility,” the authors concluded.
Reference:
Semaglutide and walking capacity in people with symptomatic peripheral artery disease and type 2 diabetes (STRIDE): a phase 3b, double-blind, randomised, placebo-controlled trial. Bonaca, Marc P et al. The Lancet, Volume 0, Issue 0
Powered by WPeMatico
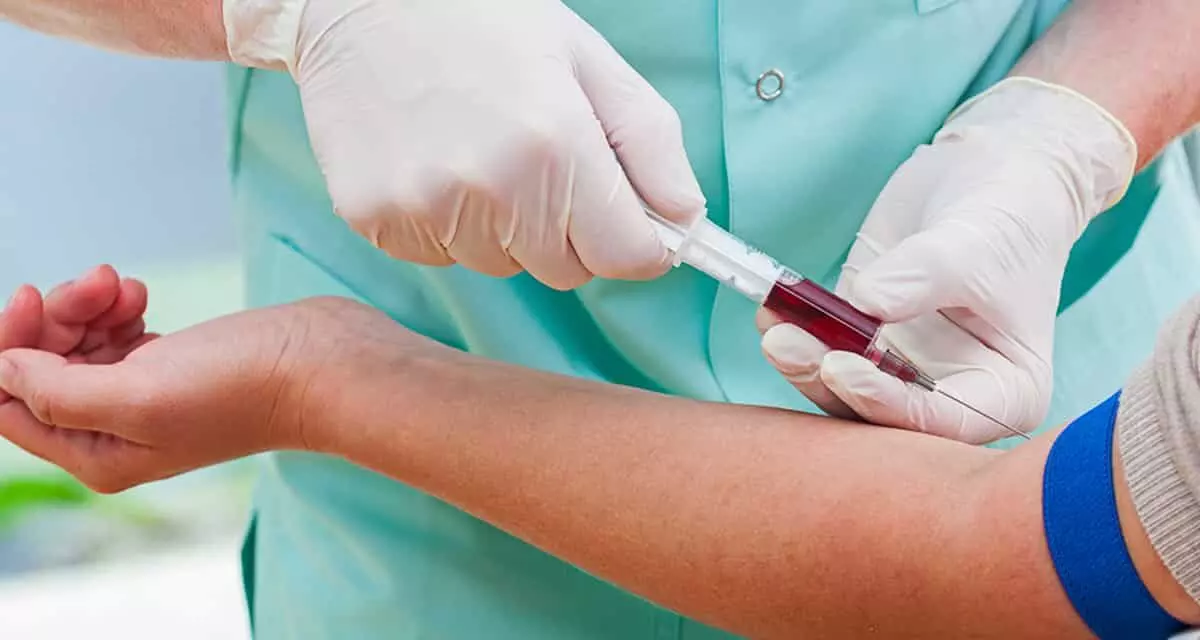
A new study published in the journal of Endocrinology, Diabetes and Metabolism showed a strong correlation between coronary artery disease (CAD) severity and an increased triglyceride-glucose (TyG) index. Atherosclerotic plaque that develops in the coronary arteries and reduces blood flow to the heart is the cause of coronary artery disease. A number of variables combine to promote atherosclerotic plaque development, which is a primary etiology that underlying the pathophysiologic processes of CAD.
Some risk factors, like smoking, diabetes, hyperlipidemia, hyperglycemia, hypertension (HTN), etc., have been shown to play crucial roles in extensive epidemiological investigations. Numerous studies have demonstrated how insulin resistance (IR) contributes to the development of atherosclerosis by causing abnormal lipid metabolism. The TyG index, which is computed as the natural logarithm of [fasting TG (mg/dL) × fasting glucose (mg/dL)/2], was created in 2008 as an affordable and easily available technique.
Global morbidity and death rates are significantly impacted by CAD, a serious public health issue. Evaluating IR as a risk factor for atherosclerosis development might reveal the pre-CAD state. A very trustworthy way to assess IR is the TyG index, which has demonstrated encouraging relationships with CAD. Thus, this study was to look at the relationship between CAD and the TyG index.
The study included a total of 2346 participants, who were divided into 5 groups, as the individuals without CAD, angiogram-negative (Ang−) patients, those with SVD, two-vessel disease (2VD), or three-vessel disease (3VD). The records were kept of biochemical analyses, illness histories, and demographic characteristics. Ln [fasting TG (mg/dL) × fasting glucose (mg/dL)/2] was used to compute the TyG index.
When compared to the healthy subjects, adjusted regression models showed that the probabilities of 3VD, 2VD, SVD, and a negative coronary angiography rose considerably with each unit rise in the TyG index. Furthermore, when compared to the Ang− group, a one-unit rise in the TyG index significantly enhanced the probabilities of becoming 3VD, 2VD, and SVD.
Overall, this study shows that the presence and severity of CAD are significantly correlated with an increased TyG index. Particularly in individuals with diabetes, higher TyG index values were consistently associated with a higher risk of multivessel CAD.
Reference:
Saffar Soflaei, S., Salehi-Sangani, P., Fallahi, Z., Imanparast, F., Marousi, M., Tajfard, M., Ferns, G. A., Moohebati, M., & Ghayour-Mobarhan, M. (2025). Triglyceride-glucose index association with severity of coronary artery disease. Endocrinology, Diabetes & Metabolism, 8(2), e70025. https://doi.org/10.1002/edm2.70025
Powered by WPeMatico
