Brensocatib may reduce frequency of pulmonary exacerbations in patients with non-cystic fibrosis bronchiectasis, uncovers phase 3 ASPEN study
Insmed Incorporated a global biopharmaceutical company on a mission to transform the lives of patients with serious and rare diseases, today announced positive topline results from the ASPEN study, a global, randomized, double-blind, placebo-controlled Phase 3 study to assess the efficacy, safety, and tolerability of brensocatib in patients with non-cystic fibrosis bronchiectasis. The study met its primary endpoint, with both dosage strengths of brensocatib demonstrating statistically significant reductions in the annualized rate of pulmonary exacerbations (PEs) versus placebo. The study also met several of its prespecified secondary endpoints with statistical significance.
Based on these results, Insmed plans to file a New Drug Application (NDA) with the U.S. Food and Drug Administration (FDA) for brensocatib in patients with bronchiectasis in the fourth quarter of 2024. Pending regulatory approvals, Insmed anticipates a U.S. launch for brensocatib in mid-2025 followed by launches in Europe and Japan in the first half of 2026. If approved, brensocatib would be the first approved treatment for patients with bronchiectasis as well as the first approved dipeptidyl peptidase 1 (DPP1) inhibitor-a new mechanism of action with the potential to address a range of neutrophil-mediated diseases.
Topline efficacy results from the ASPEN study are as follows:
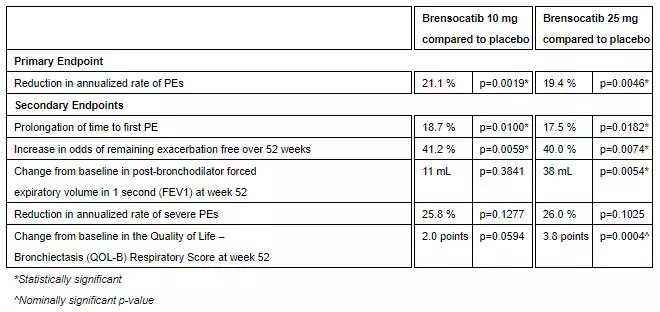
“I am thrilled that the ASPEN study has demonstrated a statistically significant and clinically meaningful treatment effect for brensocatib compared with placebo, underscoring the impact this investigational therapy may have on patients with bronchiectasis,” said lead study investigator James Chalmers, MBChB, Ph.D., Professor and Consultant Respiratory Physician at the School of Medicine, University of Dundee, UK. “Today, there is no approved treatment for bronchiectasis and there remains an urgent need for a therapy that can reduce exacerbations. As a DPP1 inhibitor, brensocatib would be the first treatment in its class and could offer a completely new approach to managing this difficult-to-treat patient population, heralding a new era in clinical management of bronchiectasis.”
As part of the ASPEN study’s conduct, more than 460 trial sites were engaged in nearly 40 countries. After excluding sites that did not enroll any patients and all sites in Ukraine, the total number of active sites in ASPEN was 391 sites in 35 countries. Adult patients (ages 18 to 85 years) were randomized 1:1:1 and adolescent patients (ages 12 to <18 years) were randomized 2:2:1 for treatment with brensocatib 10 mg, brensocatib 25 mg, or placebo once daily for 52 weeks, followed by 4 weeks off treatment. The primary efficacy analysis included data from 1,680 adult patients and 41 adolescent patients.
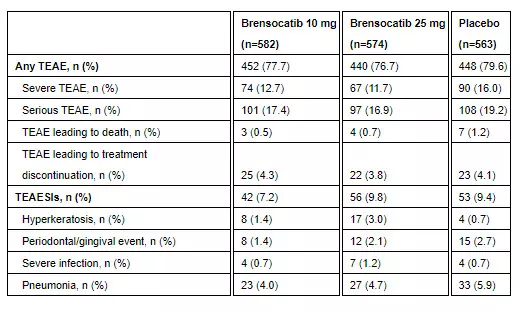
Brensocatib was well-tolerated in the study. Treatment-emergent adverse events (TEAEs) occurring in at least 5.0% of patients treated with either dose of brensocatib and more frequently than in placebo were COVID-19 (15.8%, 20.9%, 15.8%), nasopharyngitis (7.7%, 6.3%, 7.6%), cough (7.0%, 6.1%, 6.4%), and headache (6.7%, 8.5%, and 6.9%) for brensocatib 10 mg, brensocatib 25 mg, and placebo, respectively. Additional TEAEs and treatment-emergent adverse events of special interest (TEAESIs) are as follows:
“We are incredibly excited about the topline results from the pivotal ASPEN study and what they may mean for patients. These findings not only underscore our belief that brensocatib has the potential to transform the treatment landscape for bronchiectasis, but they also further validate DPP1 inhibition as a mechanism that may hold promise in other neutrophil-mediated diseases,” said Martina Flammer, M.D., MBA, Chief Medical Officer of Insmed. “Today’s outcome is the result of years of hard work and dedication by many members of the Insmed team, and I’d like to thank them for their efforts. We are especially grateful to the clinical investigators, site staff, patients, and families who made this study possible. We now look forward to further analyzing the data while rapidly advancing toward regulatory filings in our key regions, where we believe approximately 1 million bronchiectasis patients may benefit from an approved treatment.”
Brensocatib has received Breakthrough Therapy Designation from the FDA and was granted access to the Priority Medicines (PRIME) scheme by the European Medicines Agency for patients with bronchiectasis. Insmed plans to present detailed results from the ASPEN study at an upcoming medical meeting.
Insmed is also advancing the development of brensocatib in other neutrophil-driven inflammatory diseases with significant health burdens and limited treatment options. A Phase 2 study in patients with chronic rhinosinusitis without nasal polyps (CRSsNP) is currently underway, and Insmed plans to initiate a Phase 2 study in hidradenitis suppurativa (HS) in the second half of 2024.