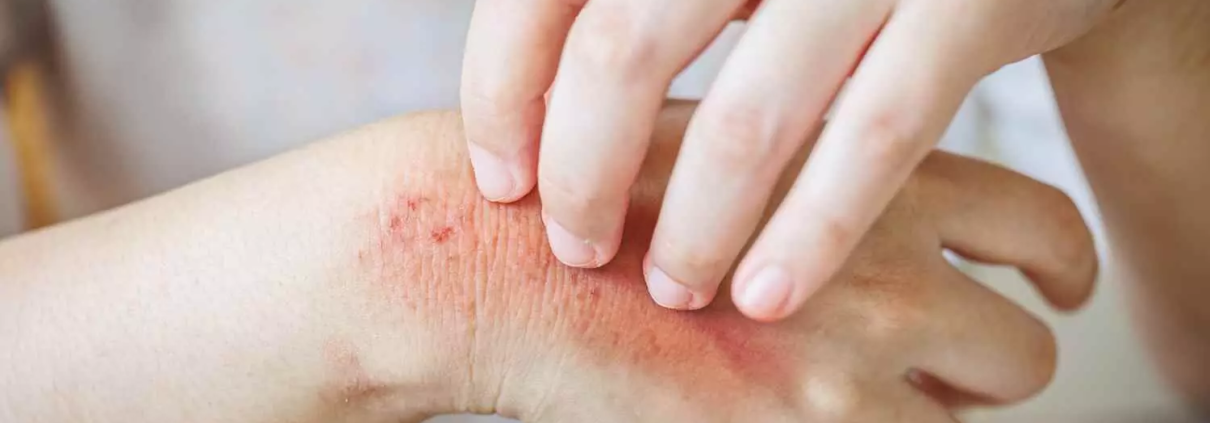
Topical gel shows promising Results for Treatment of Skin Toxicities due to cancer treatment in Phase 2a trial
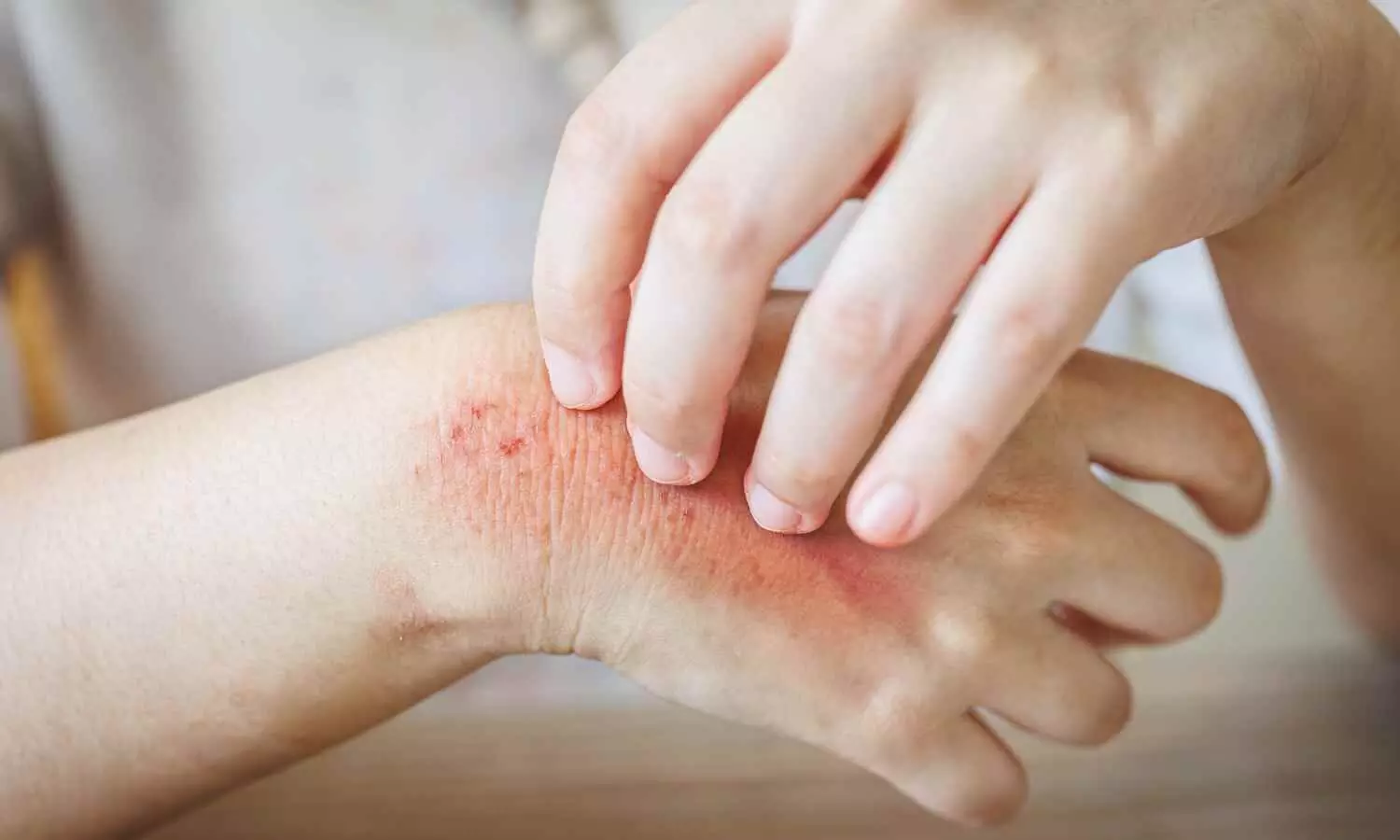
A novel topical gel, HT-001, shows promise as a treatment for cutaneous side effects caused by epidermal growth factor receptor inhibitor (EGFRi) cancer therapy. According to a press release from Hoth Therapeutics detailing phase 2a interim data, these results mark a significant milestone for addressing an unmet need in cancer treatment. In th trial 100% of patients achieved the primary endpoint of skin toxicity improvement by week 6 and 66% of patients reported a reduction in skin pain and itching.
These results highlight HT-001’s potential to set a new standard of care for cancer patients, offering a safe and effective solution to maintain cancer treatments while managing cutaneous side effects.
“These results are a significant milestone, underscoring HT-001’s potential to transform patient care by mitigating debilitating skin toxicities while maintaining critical cancer treatments,” said Robb Knie, CEO of Hoth Therapeutics. “Our data highlight HT-001’s strong safety profile and the potential for it to set a new standard of care in this underserved area.”
Exceptional Patient Outcomes:
Data from the open-label portion of the CLEER-001 trial demonstrated remarkable success:
- 100% of patients achieved the primary efficacy endpoint of an ARIGA score ≤1, showing significant skin toxicity improvement by the six-week mark.
- 66% of patients reported reduced pain and itching scores, further enhancing quality of life.
Crucially, all patients maintained their full EGFRi dosage , preserving the cancer treatment’s full therapeutic effect-a notable improvement compared to past reports showing widespread dose reductions or treatment halts due to skin-related side effects.
A Groundbreaking Approach:
The trial uses the proprietary Acneiform Rash Investigator Global Assessment Scale (ARIGA), developed in collaboration with onco-dermatology experts. The innovative scale ensures precise measurement and assessment of skin toxicity improvements.
Robb Knie , CEO of Hoth Therapeutics, remarked, “These results are a significant milestone, underscoring HT-001’s potential to transform patient care by mitigating debilitating skin toxicities while maintaining critical cancer treatments. Our data highlight HT-001’s strong safety profile and the potential for it to set a new standard of care in this underserved area.”
A Strong Safety Profile:
No treatment-related adverse effects have been reported, reaffirming HT-001’s excellent tolerability.
Looking Ahead:
“These interim findings align with a recent case report of rapid resolution of EGFRi-induced skin conditions using HT-001,” added Knie. “As the study progresses, we anticipate further validating these results and are excited about the potential impact HT-001 could have on patient outcomes.”
Hoth Therapeutics is committed to advancing groundbreaking treatments and improving patient lives through innovative therapies like HT-001.