New Clinical Trial Supports QX004N’s Safety and Effectiveness for Patients With Plaque Psoriasis
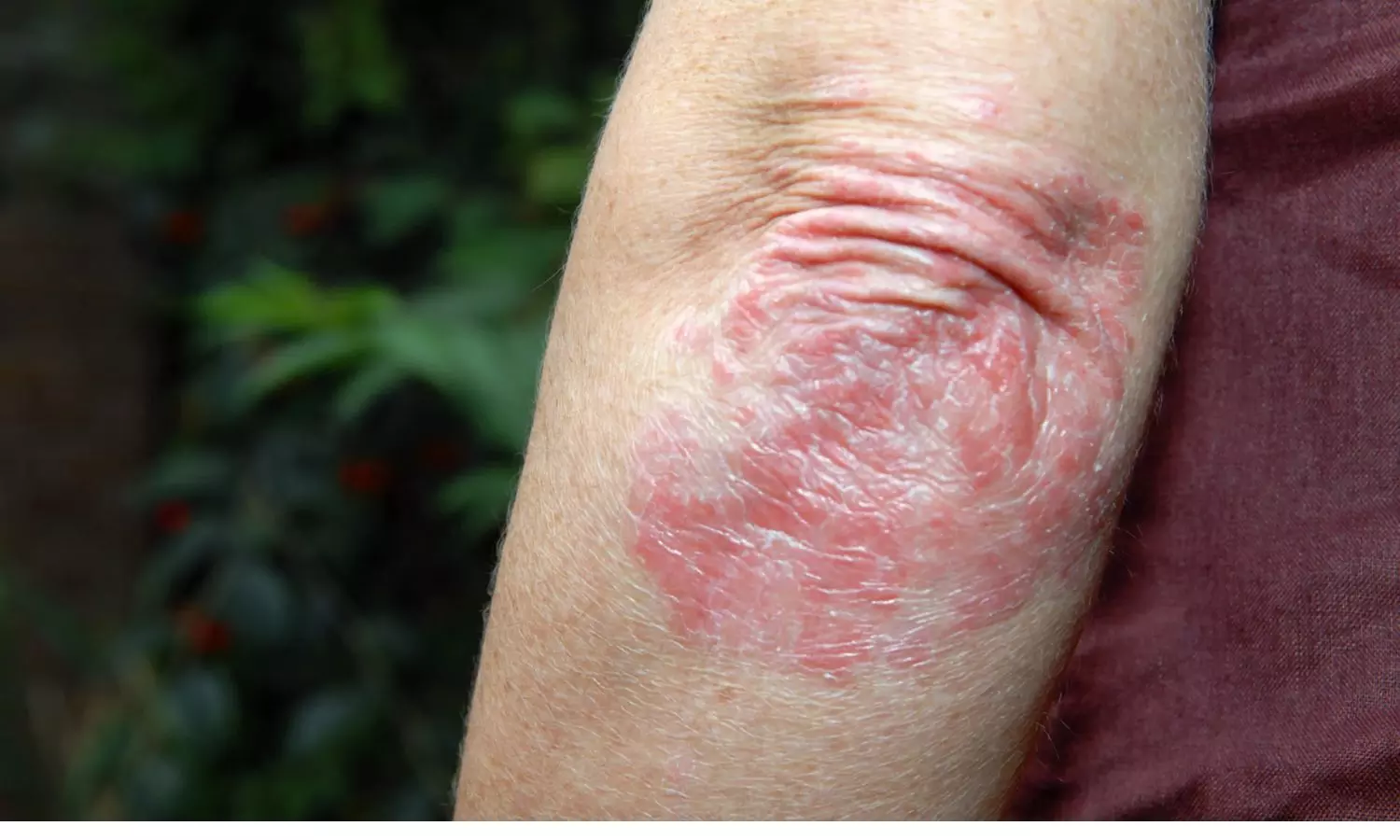
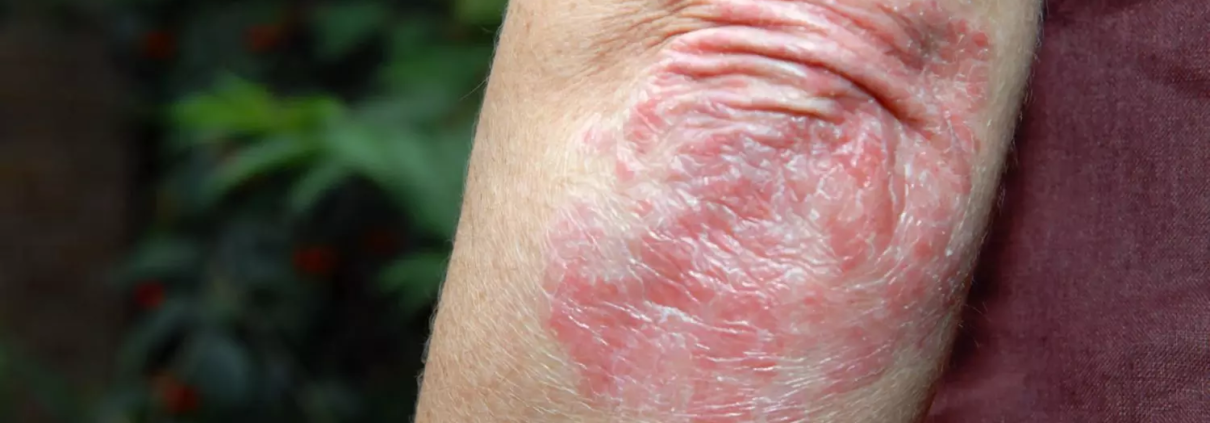
China: A new randomized clinical trial has demonstrated that QX004N, a humanized anti–IL–23 monoclonal antibody, offers superior safety and efficacy compared to a placebo in patients with moderate to severe plaque psoriasis. The findings, published in JAMA Dermatology, emphasize the potential of QX004N as a promising treatment option for individuals struggling with this chronic skin condition.
Results revealed that QX004N was well-tolerated and exhibited greater therapeutic benefit, achieving a higher rate of skin clearance than the placebo.
Psoriasis is a chronic, immune-driven skin condition with a significant need for effective biologic treatment options. IL-23 is a cytokine involved in inflammation and immune system regulation, and targeting it has been a key focus in developing new psoriasis treatments. QX004N, by inhibiting IL-23, addresses the immune dysfunction central to plaque psoriasis, aiming to reduce inflammation and control disease progression.
Against the above background, Xiaojiao Li, Phase I Clinical Trial Center, the First Hospital of Jilin University, Changchun, China, and colleagues aimed to evaluate the safety, pharmacokinetics, and efficacy of QX004N in both healthy individuals and patients with moderate to severe plaque psoriasis in China.
For this purpose, the researchers conducted a two-part randomized clinical trial in China to evaluate the safety, pharmacokinetics, and efficacy of QX004N. Part 1 was a first-in-human, single-ascending-dose phase 1a trial from November 2, 2021, to January 16, 2023, involving healthy participants receiving QX004N (10–600 mg) or placebo in a 4:1 ratio. Part 2 was a double-blind, multiple dose-escalation phase 1b trial from February 15, 2023, to January 5, 2024, involving patients with moderate to severe plaque psoriasis assigned similarly to receive 150 mg, 300 mg, or 600 mg of QX004N or placebo every two weeks.
The primary outcome of part 1 focused on safety and pharmacokinetics, while the primary endpoint of part 2 was achieving a 75% improvement in Psoriasis Area and Severity Index (PASI 75) by week 12.
The following were the key findings of the study:
- The Phase 1a clinical trial enrolled 55 healthy participants with a mean age of 35.9 years (54.5% female).
- The phase 1b clinical trial included 30 patients with moderate to severe plaque psoriasis.
- In part 2, the mean age of QX004N-treated participants was 41.4 years (79.2% male), while the mean age of the placebo group was 35.3 years (83.3% male).
- QX004N demonstrated linear pharmacokinetics and was well tolerated by healthy participants and psoriasis patients.
- Most adverse events were mild to moderate, with no drug-related serious adverse events reported.
- At week 12, 100% of patients receiving QX004N in the 150-mg, 300-mg, and 600-mg doses achieved PASI 75 (75% improvement in PASI), significantly higher than the 33.3% observed in the placebo group.
- At week 16, 100% of QX004N-treated participants achieved PASI 90 (90% improvement in PASI).
- The maximum proportion of participants achieving an Investigator’s Global Assessment score of 0 or 1 was 100% across all three QX004N dose groups.
The authors concluded that this phase 1a and 1b randomized clinical trial demonstrated that QX004N is safe and well-tolerated in healthy individuals and patients with moderate to severe plaque psoriasis in China. Treatment with QX004N at doses of 150 mg to 600 mg biweekly resulted in 100% of patients achieving a PASI 75 response by week 12, sustained through week 24, which was significantly higher than the placebo group.
“These findings support the continued development of QX004N as a promising treatment option for improving outcomes in patients with moderate to severe plaque psoriasis,” they wrote.
Reference:
Li X, Li B, Yang D, et al. Safety and Efficacy of Anti–IL-23 Monoclonal Antibody QX004N for Patients With Psoriasis: A Randomized Clinical Trial. JAMA Dermatol. Published online December 11, 2024. doi:10.1001/jamadermatol.2024.5059