Study highlights Dental Safety Concerns: Anaphylaxis Linked to PEG-Infused Endodontic Materials in dental Patients
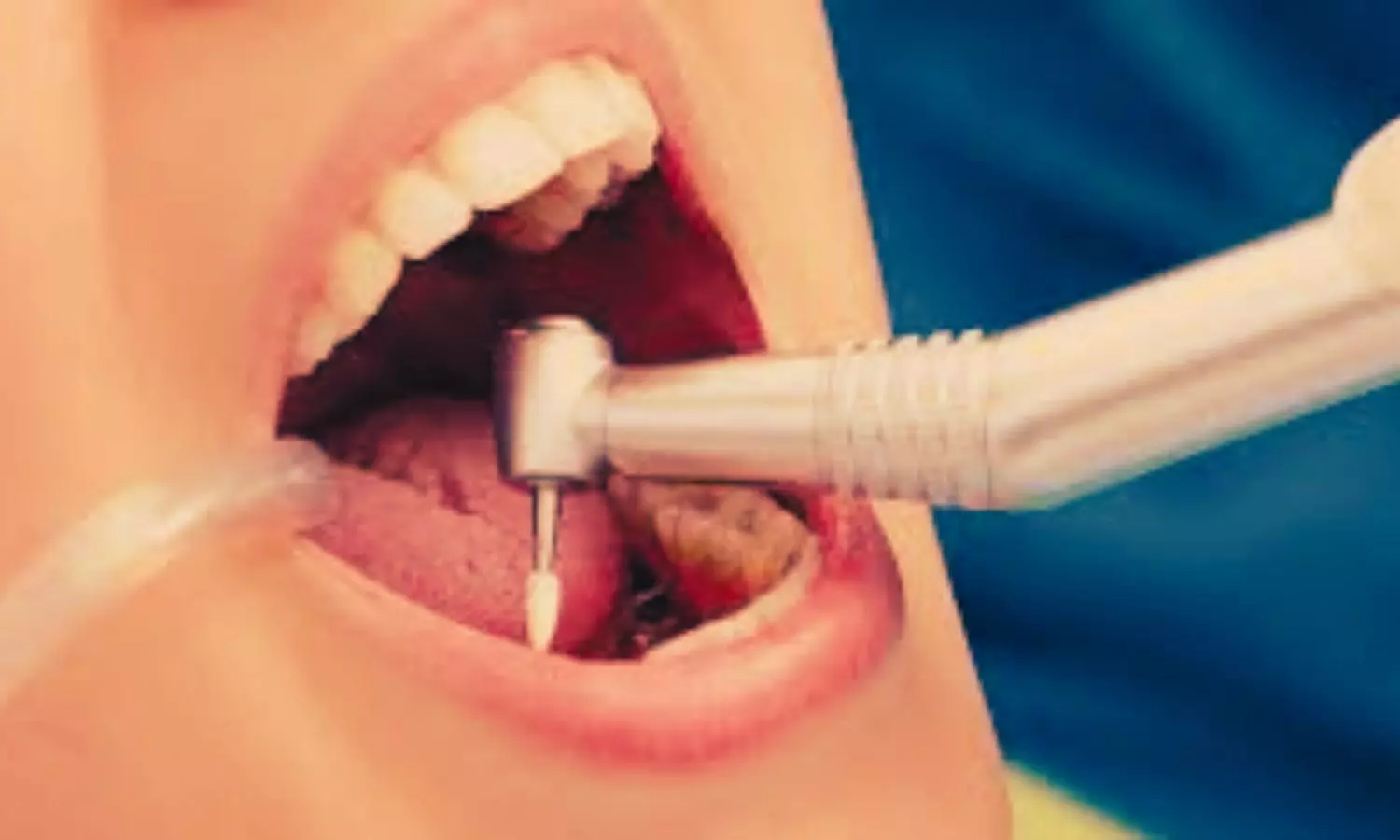
Norway: Recent case reports in the Journal of Endodontics have highlighted the rare but serious risk of anaphylaxis following treatment with endodontic materials containing polyethylene glycol (PEG). Two patients experienced severe allergic reactions after dental procedures involving these materials, raising important concerns within the dental community.
“While rare, clinicians should be aware that PEG in endodontic filling materials can trigger anaphylaxis. It is important to conduct a PEG allergy assessment when anaphylaxis is suspected,” the researchers wrote.
Anaphylaxis is a life-threatening allergic reaction that can occur rapidly, making early recognition and treatment critical. While anaphylactic reactions to PEG are uncommon, they are likely underreported and can lead to severe consequences for patients, especially those who remain undiagnosed. These cases emphasize the need for heightened awareness among dental professionals regarding the potential for PEG to trigger such reactions.
Marie Alnæs, Department of Clinical Medicine, University of Bergen, Bergen, Norway, and colleagues report two cases of anaphylaxis following the use of an endodontic temporary filling material that contains polyethylene glycol.
The first case involved a healthy man in his 30s who developed generalized itching, nausea, and cold sweats after undergoing endodontic treatment. He subsequently lost consciousness, with a blood pressure reading of 70/40 mm Hg when assessed in the ambulance. Fortunately, he recovered after receiving emergency medical care. The second case involved a patient around 50 years old, who also experienced skin itching following treatment. Tragically, this patient lost consciousness and suffered cardiac arrest upon arrival at the hospital, leading to his death.
The authors emphasize that these case reports highlight the need for increased awareness regarding the potential for endodontic materials containing polyethylene glycol (PEG) to cause anaphylaxis. They recommend that PEG allergy assessments be included in evaluations of patients experiencing anaphylaxis related to endodontic treatment. Confirmed PEG allergies can have significant implications for patients, particularly those who remain undiagnosed, as they face the risk of further anaphylactic reactions upon re-exposure.
Given the associated risks, the authors advise against using endodontic filling materials containing PEG, suggesting that alternative materials without PEG should be preferred. In cases where the benefits of using PEG-containing materials outweigh the risks, they recommend a 30-minute observation period post-treatment. Furthermore, they advocate for clear labeling in the instructions, explicitly stating that PEG in endodontic materials can trigger anaphylactic reactions.
“Thorough investigations were required to identify the source of the allergic reactions. Reports of allergies to polyethylene glycol are on the rise and can have serious consequences for patients,” the researchers concluded.
Reference:
Alnæs, M., Storaas, T., Vindenes, H. K., Guttormsen, A. B., Brudevoll, S., & Björkman, L. (2024). Anaphylaxis after treatment with an endodontic material containing polyethylene glycol. Journal of Endodontics. https://doi.org/10.1016/j.joen.2024.09.002



