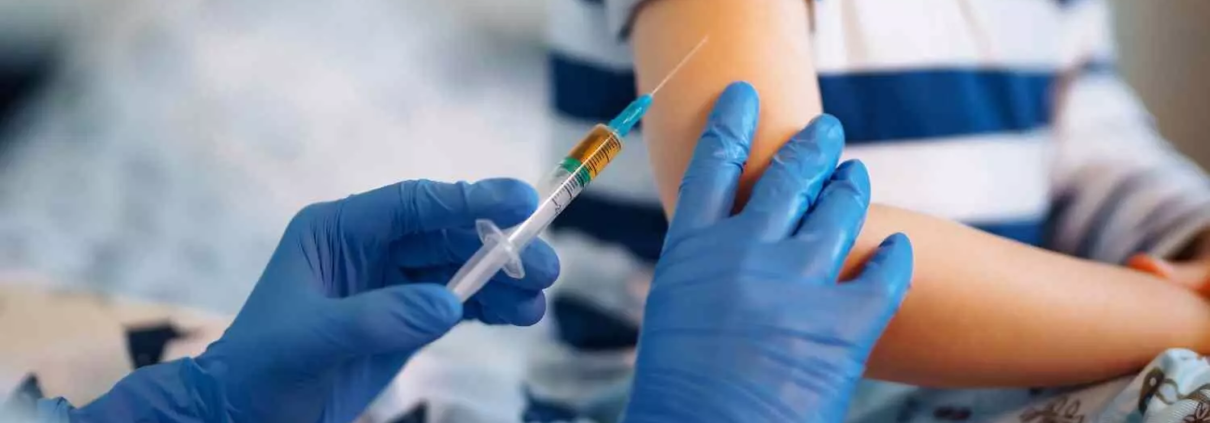
Microarray patches safe and effective for vaccinating children: Lancet

The phase 1/2 randomized trial compared results from the measles and rubella vaccine delivered by a microarray patch, a small sticking plaster-like device with an array of microscopic projections that painlessly penetrate the skin and deliver the vaccine, or by conventional injection with a needle and syringe.
The trial, which involved 45 adults (18-40 years old), 120 toddlers (15-18 months old) and 120 infants (9-10 months old) in The Gambia, found giving the measles and rubella vaccine by a microarray patch induced an immune response that was as strong as the response when the vaccine was given by conventional injection.
Over 90% of infants were protected from measles and all infants were protected from rubella following a single dose of the vaccine given by the microarray patch. The measles and rubella vaccine used in the study has been given to many millions of children globally by conventional injection and is known to provide reliable protection.
The trial found no safety concerns with delivering the measles and rubella vaccine using a microarray patch.
The trial was led by researchers from the Medical Research Council (MRC) Unit The Gambia at the London School of Hygiene & Tropical Medicine (LSHTM) and supported by the US Centers for Disease Control and Prevention. The patch was developed and manufactured by Micron Biomedical Inc, who sponsored and supported all aspects of the trial. Funding came from the Bill & Melinda Gates Foundation. Results are published in The Lancet.
The researchers hope microarray patches could help to achieve the very high levels of population immunity required to control childhood diseases such as measles and rubella, with WHO recommending at least 95% two-dose measles vaccine coverage and rubella requiring 80% population immunity. Microarray patches have been determined to be the highest priority innovation for overcoming barriers to immunization in low-resource settings.
In low-resource settings microarray patches have several advantages over conventional vaccination technologies. They promise to be easier to transport and to eliminate, or vastly reduce, the need for cold storage (refrigeration) of vaccines, both major barriers to reaching remote areas across sub-Saharan Africa. Microarray patches also do not need to be administered by a medical professional and it is expected that volunteers would be able to give the vaccines after only brief training. Unlike conventional needles and syringes, the microarray patches do not risk ‘needlestick’ injuries which can transmit infections such as hepatitis and HIV.
In countries, such as the UK, which have well-resourced childhood vaccination programmes, but which have also experienced rapid increases in measles cases recently due to low immunization coverage, microarray patches could offer greater convenience and a pain-free alternative to conventional injections. The hope is that offering vaccinations in a patch could encourage more parents, particularly those in disadvantaged areas, to get their child vaccinated.
Professor Ed Clarke, a paediatrician who leads the Vaccines and Immunity Theme at MRC Unit The Gambia at LSHTM and co-author, said: “Although it’s early days, these are extremely promising results which have generated a lot of excitement. They demonstrate for the first time that vaccines can be safely and effectively given to babies and young children using microarray patch technology. Measles vaccines are the highest priority for delivery using this approach but the delivery of other vaccines using microarray patches is also now realistic. Watch this space.”
Dr Ikechukwu Adigweme, from the Vaccines and Immunity Theme at MRC Unit The Gambia at LSHTM and co-author, said: “The positive results from this study are quite gratifying to us as a team. We hope this is an important step in the march towards greater vaccine equity among disadvantaged populations.”
The trial had several limitations. As it was the first trial to use microarray patches to deliver vaccine to children it had a small sample size and selected healthy adults, toddlers and infants. The researchers say larger trials of microarray patches are now being planned with broadly representative groups of children and infants to inform decisions about whether to recommend the patches for widespread use in childhood vaccination programmes.
Reference:
Ikechukwu Adigweme, Mohammed Yisa, Michael Ooko, Edem Akpalu, Andrew Bruce, A measles and rubella vaccine microneedle patch in The Gambia: a phase 1/2, double-blind, double-dummy, randomised, active-controlled, age de-escalation trial, The Lancet, https://doi.org/10.1016/S0140-6736(24)00532-4.