Broken sleep a hallmark sign of living with the most common liver disease, scientists find
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico

Raleigh: Accord BioPharma, Inc., the U.S. specialty division of Intas Pharmaceuticals, Ltd., focused on the development of oncology, immunology and critical care therapies, has announced that Intas Pharmaceuticals has signed an agreement to acquire the UDENYCA (pegfilgrastim-cbqv) business from Coherus BioSciences, Inc.
UDENYCA is a pegfilgrastim biosimilar to Neulasta and is typically administered the day after chemotherapy to decrease the incidence of infection as caused by febrile neutropenia. During the 2023 fiscal year, sales of UDENYCA totaled $127.1 million. “When final, the acquisition will expand Accord BioPharma’s product portfolio and is expected to position the company for accelerated growth in the biosimilar industry,” the release stated.
UDENYCA is a pegfilgrastim biosimilar approved in the United States currently available in three administration options — prefilled syringe (PFS), autoinjector (AI) and on-body injector (OBI) — providing patients and healthcare providers with choice, control and convenience. Since its launch in 2019, over 300,000 patients have been treated with UDENYCA.
“By adding UDENYCA to our growing portfolio of biosimilars, we will strengthen our business footprint in the U.S.” said Chrys Kokino, U.S. President of Accord. “With an expanding presence in the market, we can fuel the internal innovation and external expansion needed to deliver more accessible treatment options to patients.”
“In considering our strategic pathways to achieve future growth, acquiring the UDENYCA business in the U.S. is appealing for two reasons: the product’s impressive sales and uptake, and its range of administration options that give patients an opportunity to align their needs with a treatment plan,” said Binish Chudgar, Executive Chairman and Managing Director of Intas Pharmaceuticals. “Having an on-body injector within our portfolio will allow us to provide immune supportive care to patients in a variety of settings, including at home, a benefit to patients who live far away from their care center.”
The sale is expected to close in the first quarter of 2025.
UDENYCA is a leukocyte growth factor indicated to:
UDENYCA is not indicated for the mobilization of peripheral blood progenitor cells for hematopoietic stem cell transplantation.
Read also: Intas Pharmaceutical Gets CDSCO Panel Nod To Manufacture, Market Anti-cancer Drug Pertuzumab
Powered by WPeMatico

Jaipur: In a tragic incident, an MBBS student committed suicide by jumping from the hostel roof at BR Ambedkar Medical College in Udaipur. The deceased student has been identified as a second-year MBBS student.
According to IANS report, Students informed the police that the medico was studying till 2:30 in the night in his room. Officials said that he jumped around three o’clock from the sixth floor of the hostel.
Speaking to IANS, a police official said that they have taken the body in its custody and kept it in the mortuary of the district hospital.
Also Read: Final year MBBS Medico jumps to death from hostel building in Chennai
Police further informed that the student’s mobile, jacket and slippers were found on the sixth floor.
The police further informed IANS that examinations are going on in the medical college and concerned medico’s paper did not go well two days back. He had also shared about the paper with his friends.
The DSP said that on receiving the information of the incident, he reached the hospital. The body has been kept in the trauma centre.
“Medical college principal Dr Shravan Kumar Meena has given a report. We are awaiting the report of the family members. Further action will be initiated after the report comes,” the DSP said.
Medical Dialogues had earlier reported that final-year medical student of Meenakshi Medical College in Kanchipuram allegedly committed suicide by jumping from the fifth floor of her hostel building. The 23-year-old student, also a trainee doctor at the institute was reportedly suffering from depression due to relationship issues with a fellow medical student.
According to the police, the student started their relationship in the first year but gradually stopped talking and separated in their third year. This caused her extreme mental and emotional distress.
Also Read: Telangana doctor battling depression jumps to death from 8th floor of apartment
Powered by WPeMatico

Lucknow: A troubling incident has emerged in which a staff nurse employed at a private hospital in Thakurganj has accused a technician of sexual assault. The victim claims that the technician frequently made lewd comments and engaged in inappropriate behaviour towards her. Despite her efforts to resist, she alleges that he responded with verbal abuse.
The victim, a resident of Para, revealed that she had been working at the hospital for three months when she began facing persistent harassment from a technician, who hails from the Haluwapur area of Kakori.
The incident took place on November 18 at night while she was on duty. She was allegedly forced into a room and raped by the technician. Then he threatened to murder her if she told anyone about it and fled the scene. Following this the nurse filed a formal complaint at the Thakurganj police station on Saturday.
In her complaint, the nurse alleged that after reporting the incident to the hospital owners, they behaved inappropriately with her. She mentioned that after enduring the harassment by the technician she approached the hospital owners the very next day. However, her claims were reportedly dismissed by the hospital owners, who questioned her integrity and behaved indecently towards her.
The police have registered an FIR against three individuals, including the accused technician and the two hospital owners.
As per the recent media report, commenting on this case, Thakurganj police Inspector Shrikant Rai told TOI, “Nikhil has been sent to jail, while efforts are underway to apprehend the hospital owners.”
Also Read:Lucknow: 50-year-old doctor allegedly booked for raping, blackmailing tuition teacher
Medical Dialogues had earlier reported that an FIR has lodged against a middle-aged doctor for allegedly raping a teacher and blackmailing her with a videotape in which the accused recorded the act. The accused has been raping the victim for several months.
Powered by WPeMatico

Sriganganagar: In a shocking case that has drawn the attention of local authorities, a private doctor from Sriganganagar was swindled of an enormous Rs 37 lakh in a digital arrest scam. The incident, which took place four months ago, culminated in the arrest of a 24-year-old suspect on Saturday.
The ordeal began on July
16 when the doctor received a phone call from an individual posing as a Customs
Officer. The caller informed the doctor that a parcel had been intercepted,
containing 16 fake passports, 58 ATM cards, and 140 grams of MDMA drugs,
reports The Tribune.
The caller claimed that
the case had been handed over to the Delhi Police and would also involve the
Central Bureau of Investigation (CBI). Shortly after, the doctor was contacted
by another individual, identifying himself as a CBI Special Officer. Under the
guise of authority, the scammers digitally arrested the doctor, transferring Rs 36.70 lakhs from his bank account to various other accounts.
An unidentified caller
called the victim on July 16 claimed to be a Customs Officer and informed him
that the Delhi Police had taken possession of a package under his name
containing sixteen counterfeit passports, fifty-eight ATM cards, and one
hundred forty grammes of MDMA. The CBI, he added, would look into it as well,
reports the Daily.
Sub-Inspector Hansraj,
the lead investigator on the case, reported that the funds extorted from the
doctor were initially deposited into three different bank accounts before being
dispersed across approximately 18 accounts. A significant portion of the money,
amounting to Rs 5 lakhs, was traced back to an individual’s account. It is
alleged that the accused was a labourer by profession and he was manipulated by
another individual, who convinced him to sign a cheque and hand over his bank
passbook. In exchange for his cooperation, he just received a mere Rs 6,000.
As the investigation
continues, authorities are actively searching for the mastermind, who has
reportedly gone missing. The police have managed to place the Rs 5 lakhs from his
account on hold, but the majority of the fraudulent funds have already been
withdrawn by the scammers.
Powered by WPeMatico

A viral Instagram reel claims that pressing the tonsils with fingers can cure tonsils. This claim by the user is False.
In a viral Instagram reel, it is claimed that pressing the tonsils with fingers can cure tonsils. The video captioned as “अगर कोई भी टॉन्सिल या काकड़ा से परेशान है तो वीडियो को उनके साथ शेयर करो ताकि उनका भी फायदा हो सके” is posted by santosh_sharma_vlogs. In the video, the user says that in English, it’s called “tonsils,” and in Gujarati, it’s referred to as “kakda.” He further claims that Narendra Bhai treats “kakda.” According to the user, if a doctor has recommended surgery, tonsils or kakda can be treated for free here in this manner. He states, “Let me show you; if your doctor has suggested surgery, it will save your money and get the job done. Narendra is here. Here, we treated kakda tonsils without any charges. If you wish, you can come here.”
The viral reel, which has garnered 115,000 views, 25,121 likes, and 42,100 shares, is accessible here.
The claim by the user that pressing the tonsils with fingers can cure tonsils is False.
As per NIH, “The tonsils are part of the body’s immune system. Because of their location at the throat and palate, they can stop germs from entering the body through the mouth or the nose. The tonsils also contain a lot of white blood cells, which are responsible for killing germs.”
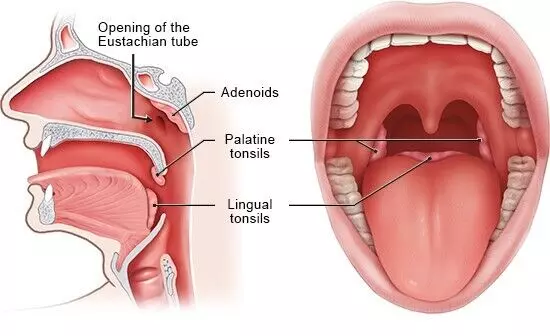
Location of the tonsils
The human body has several types of tonsils: palatine tonsils, adenoids (pharyngeal tonsils), and lingual tonsils. The palatine tonsils are located on either side of the back of the throat and are the only ones visible without specialized equipment. The adenoids are situated high in the throat behind the nose and can only be observed through rhinoscopy, a nasal examination. The lingual tonsil is found at the base of the tongue on its back surface. Collectively, these tonsillar structures form what is known as Waldeyer’s ring, encircling the throat’s entrance from the mouth and nose. This strategic positioning helps to block germs, such as bacteria and viruses, from entering the body. Additionally, immune cells located behind waldeyer’s ring can compensate for the function of removed adenoids, providing continued protection against infections
Tonsillitis is a common condition characterized by inflammation of the tonsils, accounting for about 1.3% of outpatient visits. It is primarily caused by viral or bacterial infections and often presents with symptoms such as a sore throat in uncomplicated cases.
Tonsillitis typically results from an infection, most commonly viral in origin. Viruses associated with the common cold, such as rhinovirus, respiratory syncytial virus, adenovirus, and coronavirus, are frequent causes, generally exhibiting low virulence and rarely leading to complications. Other viral pathogens like Epstein-Barr virus (mononucleosis), cytomegalovirus, hepatitis A, rubella, and HIV can also cause tonsillitis. Bacterial tonsillitis is often due to group A beta-hemolytic Streptococcus (GABHS), though other bacteria like Staphylococcus aureus, Streptococcus pneumoniae, and Haemophilus influenzae have been identified. Both aerobic and anaerobic pathogens can contribute, and in unvaccinated individuals, Corynebacterium diphtheriae (causing diphtheria) should be considered. Additionally, sexually active individuals may present with tonsillitis caused by HIV, syphilis, gonorrhea, or chlamydia. Tuberculosis has also been implicated in recurrent cases, warranting a thorough risk assessment by clinicians.

Dr Girish Anand, Consultant ENT, Head & Neck, Skull Base and Cochlear Implant Surgeon, Aster Hospital explained, “Tonsillitis refers to the inflammatory reaction that occurs to the two oval-shaped lymphoid structures situated at the back of the mouth. It is primarily due to a virus or a bacterium causing certain signs, for example, a painful throat, trouble in eating, high temperature, and lymphatic glands looking bigger. It can be recurrent and hence is subjected to assessment, further, there are therapeutic measures especially antibiotics in case of microbes or extreme conditions that may lead to tonsillectomy. Most children exhibit this condition with the exception of many age groups that this can be observed.”
The treatment of acute tonsillitis is typically managed on an outpatient basis, focusing on supportive care such as pain relief and maintaining oral hydration, with hospital admission rarely required. Symptomatic relief can be achieved using medications like steroids and nonsteroidal anti-inflammatory drugs (NSAIDs). For bacterial tonsillitis, most commonly caused by *Streptococcus pyogenes* (GABHS), penicillins are the preferred antibiotics, administered either as a 10-day oral regimen or a single intramuscular injection of benzathine penicillin G. For patients allergic to penicillin, alternative options include a 5-day course of azithromycin or a 10-day regimen of cephalosporin or clindamycin. Chronic or severe cases might also require tonsillectomy which is surgical removal.
Dr Manjunath Mk, Sr Consultant – ENT Surgeon, Gleneagles BGS Hospital, Kengeri, Bengaluru said, “Tonsillitis treatment depends on the cause (viral or bacterial) and the severity of symptoms.
Mild Cases
1. Hydration: Drink plenty of fluids to stay hydrated
2. Pain Relief: Over-the-counter medications like ibuprofen or Paracetamol
3. Saltwater gargling
If symptoms persist or worsen or if associated with fever visit ENT. It is usually treated with
1. Antibiotics. It’s important to complete the entire course of antibiotics.
2. pain medication, salt water gargling for symptomatic relief.
3. if there is a white patch or a pus points over the tonsils, this indicates a bad infection and sometimes associated with severe pain and swallowing and persistent fever for more than 48 hours. These cases are sometimes treated with injectable antibiotics. Some also require a swab to be taken.
Chronic or Recurrent Tonsillitis:
Tonsillectomy: For individuals with chronic tonsillitis or recurrent infections (usually defined as 7 episodes in 1 year, 5 per year for 2 years, or 3 per year for 3 years), a tonsillectomy (surgical removal of the tonsils) may be recommended.
If only one tonsil is enlarged and associated with severe pain and fever it may be due to pus accumulation and is known as Peritonsillar abscess and may require drainage of pus.”
Tonsils are lymphatic tissues in the throat that play a role in the immune system. They can become inflamed due to infections (tonsillitis), which are usually caused by viruses or bacteria. In ancient times, applying targeted pressure or manipulating the tonsils was thought to facilitate their treatment and removal, forming the basis for modern techniques in managing tonsillitis and improving patient care. However, in modern science and medicine, there is no medical consensus or scientific evidence to support the claim that pressing the tonsils with fingers can cure tonsils. In fact, as pointed out by doctors certain major side effects are documented with this technique
A historical study by Celsus, a Roman aristocrat, outlines a detailed method for complete tonsil removal (tonsillectomy), differentiating it from partial excision. The findings describe that the tonsils should first be carefully loosened from all sides using the finger for removal. If this method proves ineffective, the tonsils should be grasped with a blunt hook and separated using a scalpel. Post-procedure care involves washing the area with vinegar and applying a styptic to promote healing.
A study conducted in 1861 by Borelli revisited the ancient technique of tonsil enucleation using the finger. The findings highlighted Borelli’s awareness of the persistent attachment of the lower portion of the tonsil. He observed that a small fragment often remains at the inferior part, where it is challenging to grasp with the finger for removal. However, he noted that this remnant can be effectively managed by using forceps to grasp it and applying a gentle twisting motion to detach it.
In ancient medicine, some scientific evidence suggests that tonsils could be removed using fingers; however, as modern science developed, these techniques were found ineffective in the treatment of the disease and in fact were shown to have major side effects

In response to the claim Dr Manjunath Mk, Sr Consultant – ENT Surgeon, Gleneagles BGS Hospital, Kengeri, Bengaluru explained, “Pressing the tonsils as a cure is not advisable as it can cause more harm than benefit. In the acute stage, the pain becomes unbearable and in chronic cases, it can sometimes lead to severe bleeding and infection.”
Adding to the claim response, Dr Girish Anand, Consultant ENT, Head & Neck, Skull Base and Cochlear Implant Surgeon, Aster Hospital, said, “There are important questions and considerations involved in the approach of applying pressure to the tonsils with one’s fingers being a potential remedy for tonsillitis: some people might believe that manual manipulation could provide relief or healing, but it has to be remembered that the tonsils are somewhat fragile structures that can easily become irritated or injured. In fact, medical professionals usually prescribe some more traditional treatments for tonsillitis, which include rest, hydration, and sometimes antibiotics instead of simply relying on physical pressure on the tonsils. So, consulting a doctor would be a better option in order to get proper diagnosis and treatment instead of self-medication by applying finger pressure on the tonsils.”
Medical Dialogues Final Take
Tonsillitis, or inflammation of the tonsils, is caused by viral or bacterial infections and cannot be treated by physical manipulation of the tonsils. Pressing the tonsils with fingers can cure tonsils is not backed by any scientific evidence or medical consensus in modern medicine. Doctors have warned against the side effects of such techniques, pointing that they should not be applied.
Hence, the claim by the user is False.
Powered by WPeMatico
