Consumption of high amount of ultraprocessed foods impacts muscle quality, reveals research

A diet high in ultra-processed foods is associated with higher amounts of fat stored inside thigh muscles, regardless of the amount of calories consumed or level of physical activity, according to a study being presented today at the annual meeting of the Radiological Society of North America (RSNA). Higher amounts of intramuscular fat in the thigh could also increase the risk for knee osteoarthritis.
The use of natural and minimally processed ingredients in many modern diets has decreased, more often being replaced with ingredients that have been industrially processed, artificially flavored, colored or chemically altered.
Foods such as breakfast cereals, margarines/spreads, packaged snacks, hot dogs, soft drinks and energy drinks, candies and desserts, frozen pizzas, ready-to-eat meals, mass-produced packaged breads and buns, and more, include synthesized ingredients and are highly processed.
These ultra-processed foods usually have longer shelf lives and are highly appealing, as they are convenient and contain a combination of sugar, fat, salt and carbohydrates which affect the brain’s reward system, making it hard to stop eating.
For the study, researchers set out to assess the association of ultra-processed food intake and their relationship to intramuscular fat in the thigh.
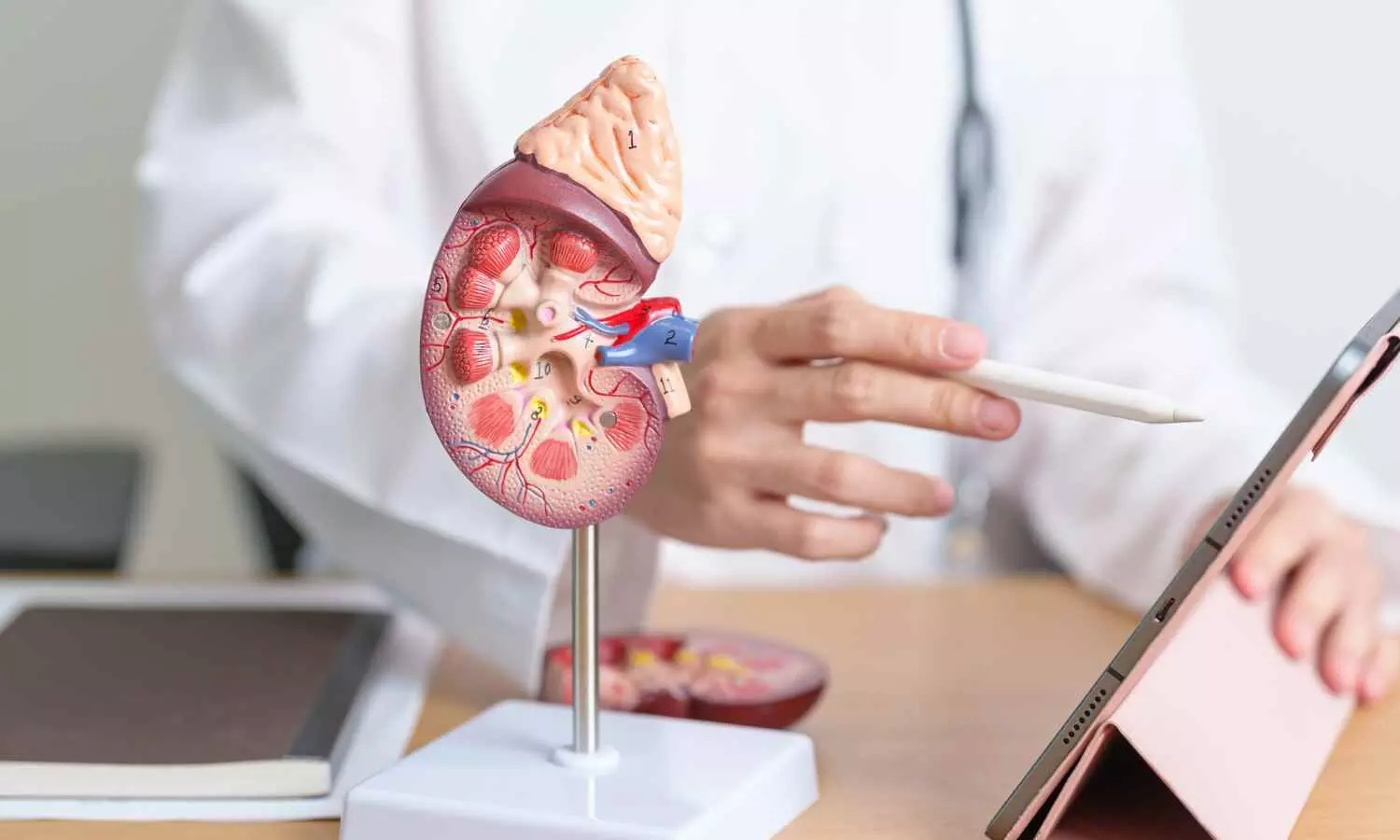
“The novelty of this study is that it investigates the impact of diet quality, specifically the role of ultra-processed foods in relation to intramuscular fat in the thigh muscles assessed by MRI,” said author Zehra Akkaya, M.D., researcher and former Fulbright Scholar in the Department of Radiology and Biomedical Imaging at the University of California, San Francisco. “This is the first imaging study looking into the relationship between MRI-based skeletal muscle quality and quality of diet.”
For the study, researchers analyzed data from 666 individuals who participated in the Osteoarthritis Initiative who were not yet affected by osteoarthritis, based on imaging. The Osteoarthritis Initiative is a nationwide research study, sponsored by the National Institutes of Health, that helps researchers better understand how to prevent and treat knee osteoarthritis.
“Research from our group and others has previously shown that quantitative and functional decline in thigh muscles is potentially associated with onset and progression of knee osteoarthritis,” Dr. Akkaya said. “On MRI images, this decline can be seen as fatty degeneration of the muscle, where streaks of fat replace muscle fibers.”
Of the 666 individuals, (455 men, 211 women) the average age was 60 years. On average, participants were overweight with a body mass index (BMI) of 27. Approximately 40% of the foods that they ate in the past year were ultra-processed.
The researchers found that the more ultra-processed foods people consumed, the more intramuscular fat they had in their thigh muscles, regardless of energy (caloric) intake.
“In an adult population at risk for but without knee or hip osteoarthritis, consuming ultra-processed foods is linked to increased fat within the thigh muscles,” Dr. Akkaya said. “These findings held true regardless of dietary energy content, BMI, sociodemographic factors or physical activity levels.”
Targeting modifiable lifestyle factors-mainly prevention of obesity via a healthy, balanced diet and adequate exercise-has been the mainstay of initial management for knee osteoarthritis, Dr. Akkaya noted.
“Osteoarthritis is an increasingly prevalent and costly global health issue. It is the largest contributor to non-cancer related health care costs in the U.S. and around the world,” Dr. Akkaya said. “Since this condition is highly linked to obesity and unhealthy lifestyle choices, there are potential avenues for lifestyle modification and disease management.”
By exploring how ultra-processed food consumption impacts muscle composition, this study provides valuable insights into dietary influences on muscle health.
“Understanding this relationship could have important clinical implications, as it offers a new perspective on how diet quality affects musculoskeletal health,” Dr. Akkaya said.
Reference:
Eating high-processed foods impacts muscle quality, Radiological Society of North America, Meeting: 110th Scientific Assembly and Annual Meeting of the Radiological Society of North America.
Powered by WPeMatico