Primary splenic pregnancy: A diagnostic and Therapeutic Enigma of Ectopic Pregnancy

A recent study found that primary splenic pregnancy, which
is a rare form of ectopic pregnancy, poses significant diagnostic and
therapeutic challenges. The study results were published in the Journal of
Obstetrics and Gynecology Research.
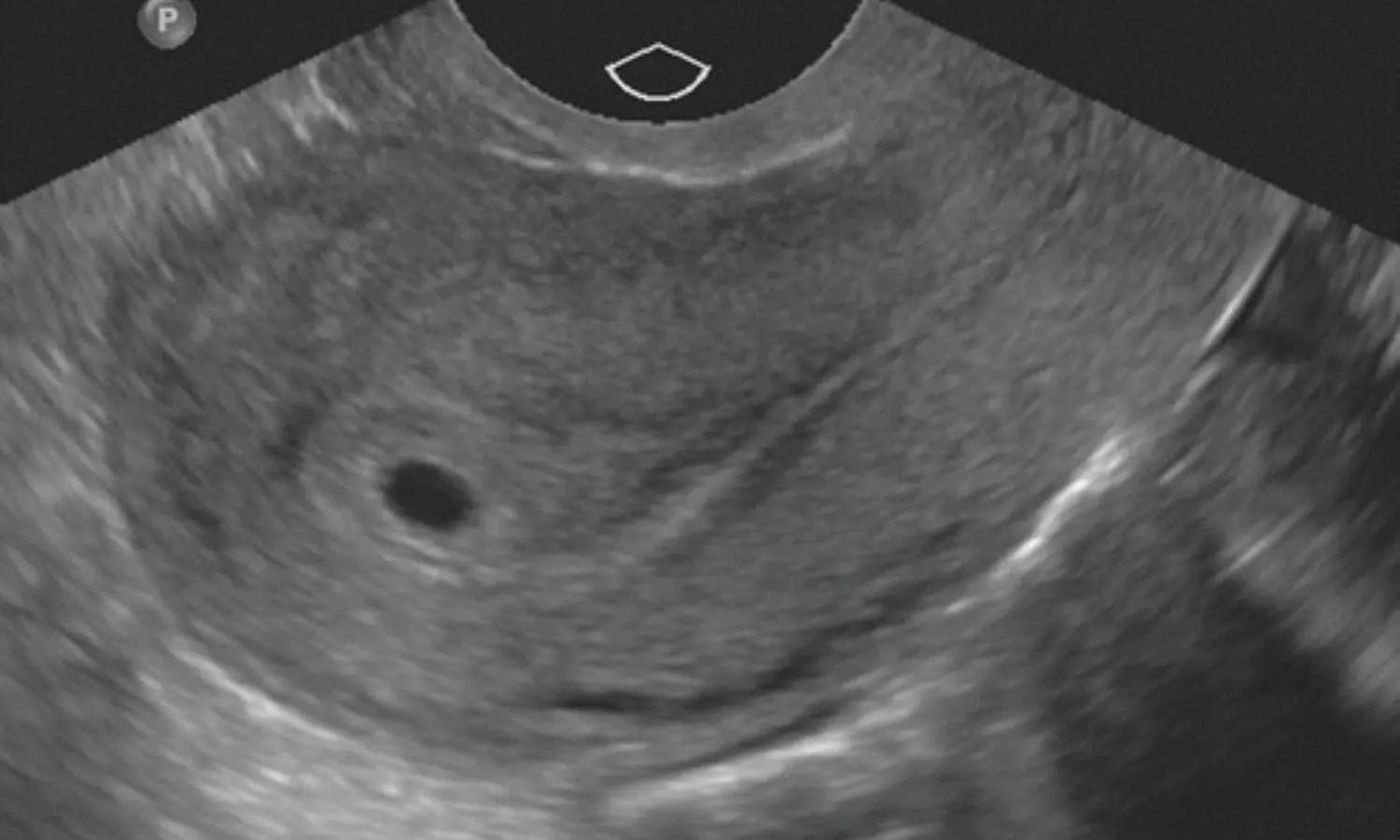
Primary splenic pregnancy is a rare type of ectopic
pregnancy which can be life-threatening. Intra-abdominal bleeding is one of the
major causes of emergency in primary splenic pregnancy. To date, about 51 cases
have been reported globally. Hence, researchers conducted a study to identify
the fundamental steps of the diagnosis necessary to reduce the mortality rate simultaneously
evaluating the available therapeutic options.
Researchers presented the diagnostic-therapeutic pathway of a
22-year-old woman with primary splenic pregnancy. To identify the best
treatment for these patients, researchers reviewed various databases in English
and all available non-English literature, including historical publications. The
collected literature was classified each article by clinical onset, diagnostic
and therapeutic strategy, and histological findings, if available.
Findings:
- About 43 cases in the English-language
literature were reviewed (plus another paper in German). - The study found that 72.7% of patients presented
in an emergency setting. - Seventy-five percent of patients required
splenectomy, 6.8% received pharmacological-only therapy, and 11.3% received
arterial embolization before definitive treatment. - The other ones received non-radical surgical
treatment.
Primary splenic pregnancy poses significant diagnostic and
therapeutic challenges due to its rare presentation. Various treatment
approaches that are used, such as pharmacological, interventional, or surgical should
be personalized based on the clinical presentation and hemodynamic stability of
the patient. The study highlighted the necessity of developing and validating
evidence-based treatment strategies to improve clinical outcomes.
Further reading: Primary splenic ectopic pregnancy: A case
report and literature review of a rare issue. Doi: https://doi.org/10.1111/jog.16154
Powered by WPeMatico