Study Links Severe Atopic Dermatitis to Increased Risk of Glaucoma Surgery

Taiwan: A recent global population-based study has highlighted a significant association between severe atopic dermatitis (AD) and an increased risk of requiring glaucoma surgery. The findings, published in the Journal of Glaucoma, suggest that individuals with both conditions, particularly those with more severe forms of AD, may face a higher likelihood of needing surgical intervention to manage glaucoma.
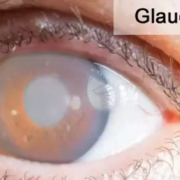
Atopic dermatitis is a chronic inflammatory skin condition that causes itching, redness, and skin lesions. While it primarily affects the skin, recent research has explored its broader impacts on other health conditions, including its potential relationship with ocular diseases like glaucoma. Glaucoma, a leading cause of blindness worldwide, is characterized by damage to the optic nerve, often caused by increased intraocular pressure. It requires long-term management, including medical and sometimes surgical interventions.
The effect of atopic dermatitis on the prognosis of glaucoma patients has been rarely explored. To address this gap, Shu-Chun Kuo, MD, from the Department of Ophthalmology at Chi-Mei Medical Center in Tainan, Taiwan, and colleagues aimed to evaluate the risk of requiring glaucoma surgery in patients with glaucoma, comparing those with and without AD.
For this purpose, the researchers conducted a retrospective cohort analysis using the TriNetX database, assessing patients with glaucoma who were initially diagnosed between December 5, 2003, and December 3, 2018. The patients were divided into two groups: those with atopic dermatitis and those without. To ensure balanced baseline characteristics and comorbidities, the researchers performed 1:1 propensity score matching.
The study compared the risk and cumulative incidence of requiring glaucoma surgery—such as minimally invasive surgery, trabeculectomy, aqueous shunt, or transscleral cyclophotocoagulation—between cohorts. A subgroup analysis was also conducted for patients with severe AD.
The study revealed the following findings:
- Out of 528,469 patients with glaucoma, 2,624 were in the atopic dermatitis group.
- Among the AD group, 584 patients had severe AD.
- The AD group showed a comparable risk of requiring glaucoma surgery to the non-AD group, with a hazard ratio of 1.03.
- In contrast, the severe AD group demonstrated a significantly higher risk and cumulative incidence of requiring surgery, with a hazard ratio of 2.80 compared to the non-AD group.
“The findings showed that patients with glaucoma and severe atopic dermatitis are significantly more likely to require surgical intervention, with the severity of AD serving as a key factor contributing to the increased risk,” the researchers concluded.
Reference:
Chu, Yung-Yu MD*; Lee, Chia-Yi MD*; Huang, Wei-Yu MD*; Lin, Ju-Kuo MD*; Liu, Ching-Chih MD*; Lin, Hsing-Ying MD*; Ho, Chung-Han PhD†,‡; Chen, Yi-Chen MS†; Kuo, Shu-Chun MD*,§. Association of Atopic Dermatitis and Risk of Glaucoma Surgery: A Global Population-Based Study. Journal of Glaucoma 33(10):p 735-741, October 2024. | DOI: 10.1097/IJG.0000000000002464
Powered by WPeMatico