Intensive glaucoma treatment may not affect visual field outcomes in patients with lower baseline high IOP: Study
Intensive glaucoma treatment may not affect visual field outcomes in patients with lower baseline high IOP suggests a study published in the American Journal of Opthalmology.
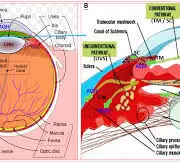
A study assessed the effect of an intensive initial IOP lowering treatment strategy on the progression of visual field damage. A total of 242 patients with newly detected early or moderate untreated open-angle glaucoma were enrolled at two university hospitals in Sweden. Participants were randomly allocated (1:1) to either initial treatment with intensive IOP-lowering medications followed by 360° laser trabeculoplasty (LTP), or to traditional mono-therapy, which was increased when deemed necessary. The primary study outcome of interest was the predicted remaining visual field, as measured by the visual field index (VFI) at projected end of life. Results: The median untreated IOP was 24 mmHg in both treatment groups. During follow-up, median and mode IOP was 17 mmHg in the mono- and 14 mmHg in the multi-treated group. In the mono-treated group the median VFI at projected end of life was 79.3%, and in the multi-treated group 87.1%, p=0.15. Annual rate of progression of visual field damage was faster in mono-treated than in multi-treated participants; median losses per year were 0.65 and 0.25 percentage units respectively, p=0.09. Progression events occurred in 21% of the mono- and in 11% of the multi-treated participants, p=0.03. Adverse events, mostly mild, were reported in 25% of the mono-, and in 36% of the multi-treated participants. Differences in visual field outcomes between treatment groups were more pronounced in participants having higher baseline IOP defined by median split of untreated IOP values. In the overall analysis the visual field outcomes were not overwhelming better in the multi-treated group, but post-hoc analysis showed definite benefit in patients with higher untreated IOP. Based upon the results of this study, initial intensive treatment may be considered in glaucoma patients with high untreated IOP at diagnosis, while we found no evidence that multi-therapy should be given routinely to all glaucoma patients.
Reference:
Bengtsson B, Heijl A, Aspberg J, Jóhannesson G, Andersson-Geimer S, Lindén C. The Glaucoma Intensive Treatment Study (GITS): a randomized controlled trial comparing intensive and standard treatment on 5 years visual field development. Am J Ophthalmol. 2024 Jun 21:S0002-9394(24)00262-9. doi: 10.1016/j.ajo.2024.06.017. Epub ahead of print. PMID: 38909742.
Keywords:
Intensive, glaucoma, treatment, affect, visual, field, outcomes, patients, lower, baseline, high IOP, study, CRT, Open-angle Glaucoma, treatment intensity, visual field, IOP, Bengtsson B, Heijl A, Aspberg J, Jóhannesson G, Andersson-Geimer S, Lindén C
Powered by WPeMatico