Single dose SelfJect corticotropin injection approved by FDA
The US Food and Drug Administration (FDA) has granted approval for supplemental New Drug Application (sNDA) for ‘Acthar Gel Single-Dose Pre-filled SelfJect Injector’ (repository corticotropin injection).
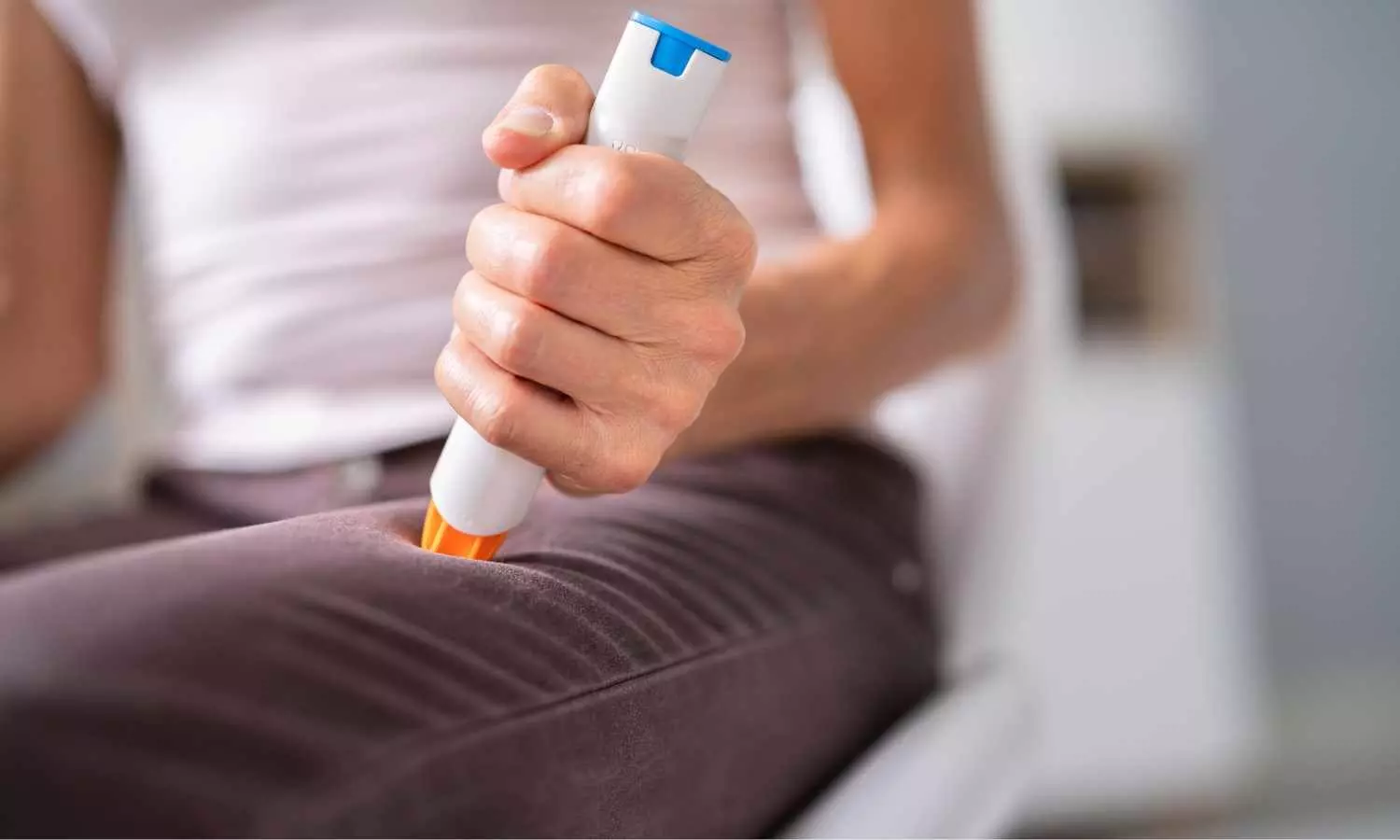
Mallinckrodt plc, announced the availability of the Acthar Gel (repository corticotropin injection) Single-Dose Pre-filled SelfJect™ Injector (herein referred to as “SelfJect”), offering a new administration option for Acthar Gel for appropriate patients with a range of chronic and acute inflammatory and autoimmune conditions.1 The U.S. Food and Drug Administration (FDA) previously approved Mallinckrodt’s supplemental New Drug Application (sNDA) for SelfJect in February 2024.
Acthar Gel is a naturally sourced complex mixture of adrenocorticotropic hormone (ACTH) analogs and other pituitary peptides.1 Acthar Gel is approved by the FDA for the treatment of several autoimmune disorders and medical conditions known to cause inflammation.
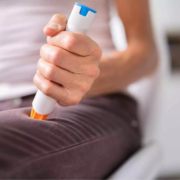
Acthar Gel is the first and only medication in its class of adrenocorticotropic hormone products available in two forms of administration – multi-dose vial and syringe and SelfJect.1 The color-coded device is pre-filled with Acthar Gel, available in 40 USP units/0.5 mL (green label) and 80 USP units/1.0 mL (purple label) versions.1,2,3 SelfJect requires less preparation with fewer materials and steps for the administration of Acthar Gel compared to the multi-dose vial and syringe.2,3 The latex-free device also has additional safety elements, including a hidden needle intended to help protect patients against needlesticks.2,3,4 SelfJect is for subcutaneous administration by people 18 years of age or older and is designed to deliver the appropriate dose of Acthar Gel, as prescribed by a healthcare professional.1,2,3
“The launch of SelfJect is a significant advancement for patients who take Acthar Gel as it is designed to simplify the injection process, help ensure accurate dosing, and has enhanced safety features. SelfJect supports patients by helping to make treatment easier to administer than a multi-dose vial and syringe, particularly for patients with dexterity issues,”5 said Kostas Botsoglou, MD, Managing Partner of Rheumatology Center of Western New York. “I’m looking forward to being able to provide this option to appropriate patients in my practice to help them adhere to their treatment plans, which are intended to better their chances for improved outcomes.”
Acthar Gel has an established efficacy and safety profile, as well as a long track record of clinical experience spanning more than 70 years.1 Acthar Gel is accessible to over 220 million individuals covered by commercial insurance and Medicare.6 Acthar Gel has been prescribed by over 9,200 healthcare professionals and used by more than 43,500 patients (2013 to 2021).7
“We’re excited to deliver an option that not only helps to address the needs of the patient communities we serve, but also underscores our commitment to the modernization of Acthar Gel. We know that managing chronic and acute inflammatory and autoimmune conditions can be difficult, and we’re proud to offer this new delivery device, designed to better support patients, caregivers, and medical professionals in managing appropriate conditions,” said Lisa French, Executive Vice President & Chief Commercial Officer.
Mallinckrodt is committed to providing therapy for appropriate patients with difficult-to-treat conditions. Mallinckrodt offers a suite of services for eligible Acthar Gel patients including support with obtaining insurance coverage, commercial copay assistance, a patient assistance program, injection training services, and customized assistance by a nurse navigator. Mallinckrodt also offers a team of field-based experts who provide education for healthcare professionals on the reimbursement process as well as tools available for patients. For more information about Mallinckrodt’s programs and patient support please visit ActharHCP.com.
For patients who prefer or require the traditional administration method, Acthar Gel continues to be available in the multi-dose vial. This method remains appropriate for patients who require doses other than 40 or 80 units.1 SelfJect is not to be used for the treatment of infantile spasms. The process for starting new patients on Acthar Gel using SelfJect remains the same as for those starting with the multi-dose vial – there are no additional access steps for SelfJect. If a new customer is interested in learning more about SelfJect, they can reach out to their local representative or visit ActharHCP.com.
INDICATIONS
Acthar Gel is indicated for:
Inducing a diuresis or a remission of proteinuria in nephrotic syndrome without uremia of the idiopathic type or that due to lupus erythematosus
Monotherapy for the treatment of infantile spasms in infants and children under 2 years of age
Treatment of acute exacerbations of multiple sclerosis in adults. Controlled clinical trials have shown Acthar to be effective in speeding the resolution of acute exacerbations of multiple sclerosis. However, there is no evidence that it affects the ultimate outcome or natural history of the disease.
Severe acute and chronic allergic and inflammatory processes involving the eye and its adnexa such as: keratitis, iritis, iridocyclitis, diffuse posterior uveitis and choroiditis, optic neuritis, chorioretinitis, anterior segment inflammation
Symptomatic sarcoidosis
Treatment during an exacerbation or as maintenance therapy in selected cases of systemic lupus erythematosus
Treatment during an exacerbation or as maintenance therapy in selected cases of systemic dermatomyositis (polymyositis)
Adjunctive therapy for short-term administration (to tide the patient over an acute episode or exacerbation) in: psoriatic arthritis; rheumatoid arthritis, including juvenile rheumatoid arthritis (selected cases may require low-dose maintenance therapy); ankylosing spondylitis
Powered by WPeMatico