Study: Rare cancer patients nearly three times more likely to develop anxiety and depression than common cancer patients
Powered by WPeMatico
Powered by WPeMatico

New Delhi: Nationwide protests have erupted in response to the rape and murder of a postgraduate trainee at R G Kar Medical College Hospital in Kolkata, leading medical professionals across India to demand a Central Protection Act for healthcare workers.
The tragic incident has spurred demonstrations at various institutions, including prominent medical colleges across the country.
AIIMS Delhi doctor Kumar Kartikay said that they will not step back till they get an assurance that action will be taken in the matter.
As per an ANI report, “We want to peacefully put forward our demands. We demand a Central Protection Act for healthcare workers. Until we get a written assurance, we will continue our strike… We are expecting around 3000-5000 people at Nirman Bhawan today from all the medical colleges in Delhi… We will not step back or sit quietly till we get an assurance that action will be taken,” he said.
Similarly, junior doctors and medical students from AIIMS Mangalagiri in Andhra Pradesh have taken to the streets, performing a street play to highlight the plight of the victim from RG Kar Medical College and Hospital in Kolkata.
Dr Sreeja, a Senior Resident Doctor at AIIMS Mangalagiri, has called for a transparent investigation by the CBI and urged that the victim’s family receive adequate compensation.
“On the eve of Independence Day, protesting doctors at RG Kar faced mob attacks and also police attacks. The hospital was vandalised and evidence was erased. In condemnation of this, we are protesting here… It’s not just a crime against doctors. It’s a crime against women and humanity… We request the CBI to conduct a transparent investigation… The victim’s family should receive adequate compensation… We also demand a Central Protection Act for all healthcare workers,” she said, news agency ANI reported.
Medical professionals at Chandigarh PGIMER have been continuing the protest on Friday. The doctors have stated that the protest will continue till justice is delivered.
Patients who have been coming to the Chandigarh PGIMER stated that they have been facing a lot of trouble because of the ongoing protest for five days and urged the government to fulfil the demands of the doctors.
“We are facing a lot of problems due to the absence of OPD. We have come from a long distance,” said Nitin, a patient, adds ANI.
“If the OPD is closed, then, we should have informed first. Now we have come. Our checkup was done on the old card but the card for meeting the new doctor is not being made, so we will have to come again,” said another patient Pushpa.
“We have been roaming around here since last night but no one is listening to us nor the doctors are attending to us. The government must listen to the doctors so that the strike gets over,” said Gurpreet, a patient.
Meanwhile, the Resident Doctors Association, Government Medical College in Amritsar, Punjab has announced the suspension of all non-essential and elective hospital services, including Out Patient Departments, Operating theatres and wards, beginning on August 16 and continuing until further notice.
Powered by WPeMatico

Mumbai: Global pharma major Lupin Limited has announced that the Company has received tentative approval from the United States Food and Drug Administration (U.S. FDA) for its Abbreviated New Drug Application for Brimonidine Tartrate Ophthalmic Solution, 0.025% (OTC), to market a generic equivalent of Lumify Ophthalmic Solution, 0.025%, of Bausch & Lomb Incorporated. This product will be manufactured at Lupin’s Pithampur facility in India.
Brimonidine Tartrate Ophthalmic Solution, 0.025% (OTC) is indicated to relieve redness of the eye due to minor eye irritations.
Brimonidine Tartrate Ophthalmic Solution (RLD Lumify) had an estimated annual sale of USD 39 million in the U.S. (IQVIA MAT June 2024).
Read also: Lupin Announces Positive Results from Global Phase 3 Trial of Lucentis Biosimilar
Medical Dialogues team had earlier reported that the Company had received approval from the U.S. FDA for its ANDA for Prednisolone Acetate Ophthalmic Suspension USP, 1% to market a generic equivalent of Pred Forte Ophthalmic Suspension, 1%, of AbbVie Inc
Read also: Lupin bags USFDA nod for Prednisolone Acetate Ophthalmic Suspension
Lupin Limited is a global pharmaceutical leader headquartered in Mumbai, India, with products distributed in over 100 markets. Lupin specializes in pharmaceutical products, including branded and generic formulations, complex generics, biotechnology products, and active pharmaceutical ingredients. Trusted by healthcare professionals and consumers globally, the company specializes in India and the U.S. across multiple therapy areas, including respiratory, cardiovascular, anti-diabetic, anti-infective, gastrointestinal, central nervous system, and women’s health. Lupin has 15 state-of-the-art manufacturing sites and 7 research centers globally.
Powered by WPeMatico

New Delhi: 27-year-old Dr RG, presented to ENT OPD in an emergent condition with complaints of acute onset vision loss in right eye for 3 days.
He also has history of right-sided nasal obstruction off and on since the last 3 months.
He underwent a CT scan of his sinuses, and he was diagnosed to have nasal polyposis and fungal sinusitis which was compressing the nerve of vision inside the sphenoid sinus. This is a rare condition and if not acted upon soon can lead to complete blindness in the affected eye.
Also Read:Sir Ganga Ram Hospital Doctors use E-CPR to save 11-year-old girl with Viral Myocarditis
He was administered IV antibiotics and steroids which improved the signs of compression on the nerve. He underwent Emergency Sinus surgery with Endoscopic Optic Nerve Decompression.
During the surgery, fungus was seen to be spreading in all sinuses and between the nerve and the brain layer in the sphenoid sinus. Complete removal of the disease was achieved, and the nerve was decompressed and freed from the surrounding swollen nasal lining.
Dr Varun Rai stated that these types of surgeries are extremely specialised due to the fact that the optic nerve is very delicate and a slightest misstep can lead to permanent blindness. He used special instruments around the nerve so as not to cause pressure over the affected part of nerve while still achieving complete clearance of fungal tissue around it. The nerve was then decompressed 270 degrees leaving the blood supply to the nerve intact.
In the immediate post operative period his vision improved and gradually returned to normal within 2 days. He was discharged in a stable condition in two days with vision which was completely restored.
Dr Varun Rai, ENT Consultant, SGRH said, “This case serves to highlight the rarity of the condition and the fact that untreated sinusitis can lead to consequences which can have devastating complications. “
He also mentioned that this case was unique in the sense that the anatomy of the sphenoid sinus was such that it allowed the fungus to creep in between the optic nerve and the brain dura layer leading to compression and eventually blindness. He also stated that prompt treatment is necessary in such cases and surgeons typically have only a few hours to save the eye in cases of complete blindness.
Dr Manish Munjal, ENT Senior Consultant, SGRH opined that these cases are rare nowadays due to increased awareness and good diagnostic facilities which are readily available. He also mentioned the increased incidence of such cases due to rising pollution levels which leads to nasal allergic responses.
The typical symptoms which patients present with are nasal stuffiness and discharge from the nose. The ENT doctor establishes a diagnosis based on an OPD based endoscopic examination and a CT scan.
Sir Ganga Ram Hospital has been at the forefront of innovation and technology and has recently acquired the Image Guided Navigation system where we routinely use real time surgical mapping based on preoperative CT scans to perform nasal surgery in a safe and effective manner. This ensures complete disease clearance in all patients with minimal chances of complications during surgery. SGRH was the first private Hospital in Delhi to start the Navigation Guided Sinus surgery program and has been leading the way in training and teaching programmes around the country.
Powered by WPeMatico

Chhattisgarh- The Directorate of Medical Education (DME), Chhattisgarh is going to begin the NEET counselling registrations tomorrow. All the concerned candidates are advised to take note of the application information released by the DME as mentioned below.
Online applications have been invited by the DME for admission to the
undergraduate courses (MBBS/BDS) conducted in government and private medical
colleges and dental colleges of Chhattisgarh state for the year 2024.
Here are the application details:
|
No. |
Process Details |
Description |
|
1. |
For |
1.Online 2. For 3. For Non |
|
2. |
Registration |
See the |
|
3. |
Online |
Date 18 |
|
4. |
Online |
Date 24 |
|
5. |
Choice |
1. Date 18 2. From 25 |
After the first allotment of MBBS, only the previously
applied eligible candidates will be given the opportunity to choose the
institution again before the second counselling allotment (the previous
institution choice will become void) and only the institution choice will be
changed in the second counselling.
Petition WPC No. filed in Honorable High Court, Bilaspur.
1632 of 2022 Prachi Sharma D/o Shri Mukesh Sharma Vs State Of Chhattisgarh. WPC
No. To avoid legal complications in counseling due to 1691 of 2022 Mayank Kumar
Pandey vs Union of India, through the Secretary and others and other cases,
like previous years, this year too, like All India Online Application, Medical
and Dental Undergraduate Course (MBBS/ Online application fee and registration
amount (Security Deposit Money) will have to be deposited at the time of online
application for BDS) counselling.
Registration Fee / Security Deposit: – This amount is
refundable, but if you do not take admission in the second counseling after
allotment, and if you give up the seat after taking admission, then this amount
will be forfeited. Will be done. This registration amount (security deposit
amount) is not applicable in BDS course.
(Only for registration amount see: Gazette of India
Extraordinary Indian Medical Council Amendment Notification New Delhi, May 18,
2018 No. IPC-34 (41) / 2018-Med/109835, Table No. 4 of Rule 5)
|
Category |
Registration amount |
private college or Registration amount |
|
UR |
100000/- (Rs. |
10000/- (Rs. |
|
SC/ST/OBC |
5000/- (Rs. |
100000/- (Rs. |
Necessary
information for online registration:
1. Before registering online, study the Chhattisgarh
Medical, Dental and Physiotherapy Undergraduate Admission Rules, 2018 and
Information Bulletin completely, especially the reservation/counselling
process/bond/disabled persons/other cadre etc.
2. Even if after completing the online application you have
locked & submitted it, you can still make changes in the online
application.
If you want to make changes, the facility to reset for free
will be available till the last date of application. 3. Keep visiting the
websites daily for counselling
process and other information.
4. After the release of counselling related information,
there is a possibility of less time for scrutiny and admission, hence regularly
visit the website www.cgdme.admissions.nic.in and www.cgdme.in for scrutiny and
admission of all the documents. See the procedure, observe Rule 7 (XXI) of
Undergraduate Admission Rules 2018 and keep all the required documents
available till the last date of online application (See Rules of Chhattisgarh
Medical, Dental and Physiotherapy Undergraduate Admission Rules-2018) 7(iii)).
5. If there is any error in the online application (like
incomplete application/non-deposit of registration fee through the gateway,
etc.) you become ineligible. Therefore, completing the process 24 hours before
the last date of online application is a safe process.
6. Internet Banking / Debit Cards & Credit Card, Options
are available, are available for fee deposit. Candidates are advised to pay the
application fee and registration fee (security deposit) through the candidates
own bank account or their parent/guardian’s bank account. Additionally, they
will also have to provide an alternate bank account number in case of any
contingency. If the candidate is eligible for refund as per rules, then the
registration amount (security fund amount) will be refunded to the deposit
account only from which the registration fee has been paid by the candidate.
7. Along with the primary bank account, it is advisable to
keep the alternate bank account also in active mode. In case of any unavoidable
and technical reasons, refund is made to the primary bank account, the refund
will be made to the alternate active bank account provided by the candidate on
the registration portal.
8. According to Rule 7 (X) of Chhattisgarh Medical, Dental
and Physical Therapy (Physiotherapy) Undergraduate Admission Rules 2018,
“After allotment, the candidates should present themselves in the allotted
institution by the prescribed last date of admission mentioned in the allotment
letter and submit the documents. It will be mandatory to get the scrutiny done
in case the candidate does not appear for scrutiny in the institution in the
current academic session. It will be mandatory to take admission in Government
Medical College, but in Private Medical College/Government Dental College. It
is not mandatory to take admission in college/private dental college, but it is
mandatory for all allotted candidates to undergo scrutiny and be eligible in
scrutiny.
9. If allocation is made in the second counselling in
Government/Private/Non-Resident Indian (NRI) seat, and admission is not taken
by the applicant. The registration fee (security fund amount) deposited will be
forfeited.
10. The entire process of counselling (payment of
registration amount, selection of institution and subject, choice locking etc.)
is done by the candidate himself, hence in case of providing incomplete or
wrong information, if the registration or allotment of the candidate is
cancelled, then his The entire responsibility will be solely of the candidate
and this office or the counselling committee will not be responsible in any
way.
To view the Application Click the link below
https://medicaldialogues.in/pdf_upload/14-08-2024-neet-ug-2024-online-application-notice–248089.pdf
Powered by WPeMatico

High intake of animal-based fat including fat from dairy foods and eggs associated with elevated risk for both overall and CVD mortality suggests a new study published in the JAMA Internal Medicine.
The impact of dietary fat intake on long-term human health has attracted substantial research interest, and the health effects of diverse dietary fats depend on available food sources. Yet there is a paucity of data elucidating the links between dietary fats from specific food sources and health. A study was done to find associations of dietary plant and animal fat intake with overall mortality and cardiovascular disease (CVD) mortality. This large prospective cohort study took place in the US from 1995 to 2019. The analysis of men and women was conducted in the National Institutes of Health–AARP Diet and Health Study. Data were analyzed from February 2021 to May 2024. Results The analysis included 407 531 men and women (231 881 [56.9%] male; the mean [SD] age of the cohort was 61.2 [5.4] years). During 8 107 711 person-years of follow-up, 185 111 deaths were ascertained, including 58 526 CVD deaths. After multivariable adjustment (including adjustment for the relevant food sources), a greater intake of plant fat (HRs, 0.91 and 0.86; adjusted ARDs, −1.10% and −0.73%; P for trend < .001), particularly fat from grains (HRs, 0.92 and 0.86; adjusted ARDs, −0.98% and −0.71%; P for trend < .001) and vegetable oils (HRs, 0.88 and 0.85; adjusted ARDs, −1.40% and −0.71%; P for trend < .001), was associated with a lower risk for overall and CVD mortality, respectively, comparing the highest to the lowest quintile.
In contrast, a higher intake of total animal fat (HRs, 1.16 and 1.14; adjusted ARDs, 0.78% and 0.32%; P for trend < .001), dairy fat (HRs, 1.09 and 1.07; adjusted ARDs, 0.86% and 0.24%; P for trend < .001), or egg fat (HRs, 1.13 and 1.16; adjusted ARDs, 1.40% and 0.82%; P for trend < .001) was associated with an increased risk for mortality for overall and CVD mortality, respectively, comparing the highest to the lowest quintile. Replacement of 5% energy from animal fat with 5% energy from plant fat, particularly fat from grains or vegetable oils, was associated with a lower risk for mortality: 4% to 24% reduction in overall mortality, and 5% to 30% reduction in CVD mortality. The findings from this prospective cohort study demonstrated consistent but small inverse associations between a higher intake of plant fat, particularly fat from grains and vegetable oils, and a lower risk for both overall and CVD mortality. A diet with a high intake of animal-based fat, including fat from dairy foods and eggs, was also shown to be associated with an elevated risk for both overall and CVD mortality.
Reference:
Zhao B, Gan L, Graubard BI, et al. Plant and Animal Fat Intake and Overall and Cardiovascular Disease Mortality. JAMA Intern Med. Published online August 12, 2024. doi:10.1001/jamainternmed.2024.3799
Powered by WPeMatico

Mumbai: Sun Pharmaceutical Industries Limited has entered into an agreement with Pharmazz Inc. Delaware US, pursuant to which the Company has agreed to invest upto USD 15 million resulting in more than 5 percent holding in Pharmazz Inc.
Sun Pharma shall also receive the exclusive right to license Sovateltide for marketing & distribution in certain emerging market countries.
As per the filing, USD 7.5 million (Tranche 1) shall be invested by August 2024, while USD 7.5 million (Tranche 2) shall be invested by February 2025, subject to fulfillment of certain conditions.
The investment shall be convertible to equity at 20 percent discount to pre-money valuation of next qualified fund raise by Pharmazz, subject to premoney valuation cap of USD 130 Million.
Sun Pharma is an Indian multinational pharmaceutical company headquartered in Mumbai, Maharashtra. The company manufactures and markets a large basket of pharmaceutical formulations covering a broad spectrum of chronic and acute therapies. It includes generics, branded generics, specialty, complex or difficult-to-make technology-intensive products, over-the-counter (OTC), antiretrovirals (ARVs), Active Pharmaceutical Ingredients (APIs), and Intermediates.
Powered by WPeMatico
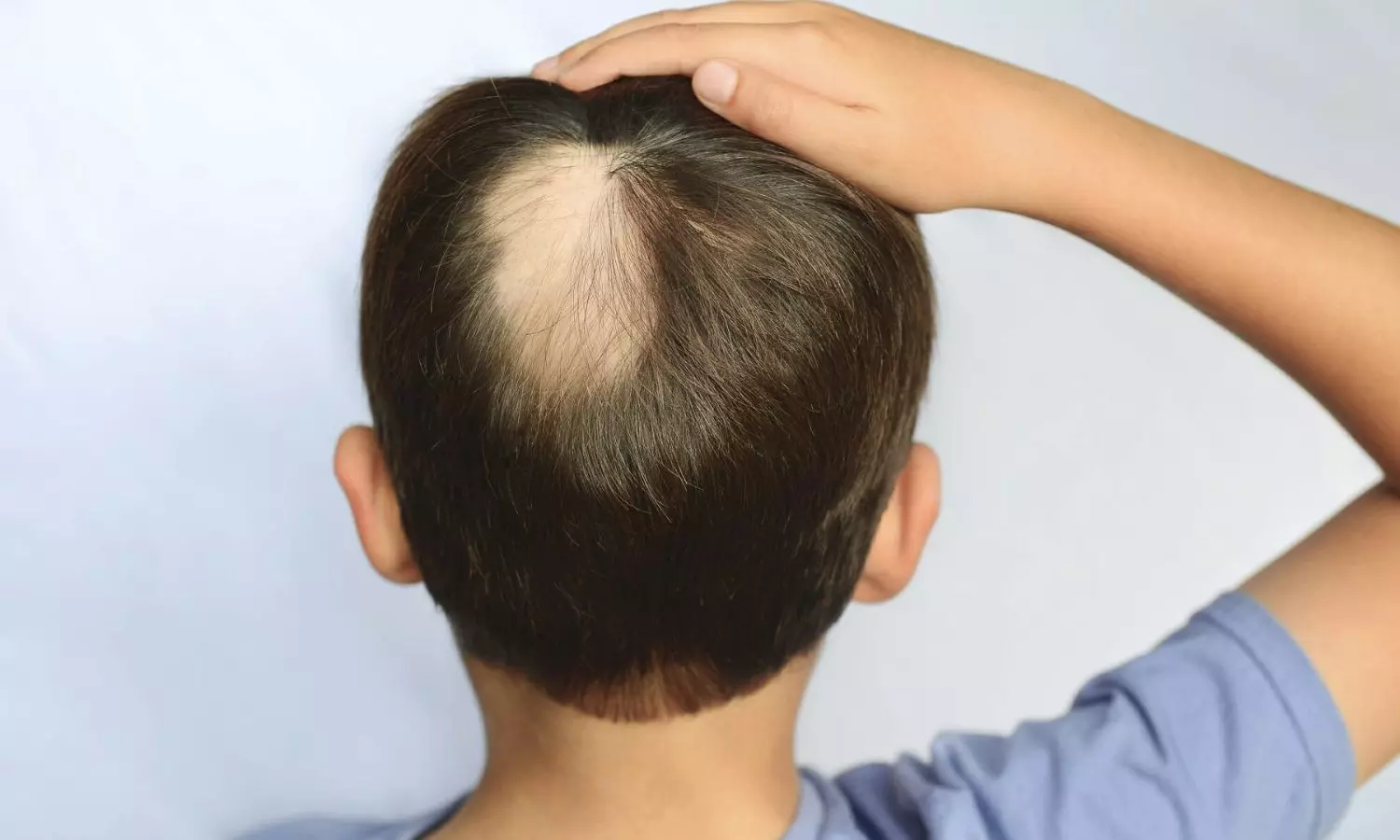
USA: A real-world, single-center observational study revealed that dupilumab significantly enhanced hair regrowth in pediatric patients with alopecia areata (AA) and atopic dermatitis, suggesting it may be a safe and promising long-term treatment option.
“The real-world data affirm the effectiveness of dupilumab for safely treating pediatric patients with alopecia areata (AA), supporting the involvement of Th2 skewing in children with AA and associated atopy. This underscores the need for larger clinical trials to further explore these findings,” the researchers wrote in Archives of Dermatological Research.
Alopecia areata is a nonscarring form of hair loss marked by Th1 and concurrent Th2 skewing, especially in atopic individuals. While there have been advances in treating adult AA, safe and effective options for pediatric patients are still lacking. Dupilumab, known for its well-established safety profile, could offer promising therapeutic potential for pediatric patients with atopic alopecia areata.
Against the above background, Emma Guttman-Yassky, Department of Dermatology, and Laboratory of Inflammatory Skin Diseases, Icahn School of Medicine at Mount Sinai, New York, NY, USA, and colleagues aimed to evaluate the ability of dupilumab to regrow hair in pediatric AA patients.
For this purpose, the researchers conducted a single-center, retrospective observational study to assess hair regrowth using the Severity of Alopecia Tool (SALT) in 20 children aged 5 to 16 years (mean age 10.8 years) with both atopic dermatitis (AD) and alopecia areata (AA). The baseline SALT scores ranged from 3 to 100, with a mean of 54.4.
The study collected data on patient demographics, atopic history, IgE levels, and SALT scores during follow-up visits at 12 weeks and up to more than 72 weeks to evaluate hair regrowth. Spearman correlations were used to analyze the relationship between clinical data and hair regrowth outcomes.
The study led to the following findings:
The study has several limitations, including its retrospective design, relatively small sample size, and absence of a comparator group. Despite these constraints, it represents the largest cohort of pediatric alopecia areata patients treated with dupilumab over an extended follow-up period.
“Future research, including larger randomized placebo-controlled trials, is needed to further assess the benefits of dupilumab for the long-term management of AA in children and adolescents with concurrent atopy and familial atopic conditions,” the researchers concluded.
Reference:
David, E., Shokrian, N., Del Duca, E. et al. Dupilumab induces hair regrowth in pediatric alopecia areata: a real-world, single-center observational study. Arch Dermatol Res 316, 487 (2024). https://doi.org/10.1007/s00403-024-03225-4
Powered by WPeMatico
The traditional lipid panel may not give the full picture of cholesterol-related heart disease risk for many Americans, according to a study led by UT Southwestern Medical Center researchers and published in JAMA Cardiology.
There are different types of cholesterol particles that can cause heart disease, including low-density lipoproteins (LDL), very low-density lipoproteins (VLDL), and intermediate-density lipoproteins (IDL). LDL-C is a measure of the weight of cholesterol in LDL particles and is one of the most common tests people use to measure cholesterol risk. Every LDL, VLDL, and IDL particle has a single protein on its surface called apolipoprotein B (apoB).
Prior research has shown that the number of “bad” cholesterol particles, measured by a blood test for apoB, is the most accurate marker for cholesterol risk. However, current guidelines do not recommend testing for apoB in all people. Instead, most only have their LDL-C measured, but that does not test for the total number of LDL particles. Measuring LDL-C alone may not be adequate to find people with high apoB levels, UTSW researchers and colleagues said.
“For most patients, the LDL-C measurement is usually ‘good enough’ because people with high LDL-C also usually have high apoB and vice versa, but that’s not true for everyone,” said senior author Ann Marie Navar, M.D., Ph.D., Associate Professor of Internal Medicine in the Division of Cardiology and in the Peter O’Donnell Jr. School of Public Health at UT Southwestern. “Some people have high apoB but a relatively low LDL-C, so their heart disease risk is underestimated by not measuring apoB. Others may have a high LDL-C but a low or normal apoB, and they aren’t at risk.”
Because the weight of cholesterol particles can vary from person to person, LDL-C and apoB measurements don’t always line up. When apoB levels vary from estimated values, they’re called “discordant.” In the case of patients with low or normal-appearing LDL-C and a high apoB level, LDL-C measurements may offer a false sense of security. This happens more commonly in people with metabolic risk factors such as obesity, diabetes, or high triglycerides. But even people without these conditions can have discordance.
The research team used data from the National Health and Nutrition Examination Survey (NHANES) to assess apoB discordance in the U.S. population. The NHANES database included apoB, LDL-C, high-density lipoprotein cholesterol (HDL-C, or “good” cholesterol), total cholesterol, and triglyceride levels for 12,688 adults measured between 2005 and 2016. To determine the discordance level for each individual, Dr. Navar and her colleagues calculated the difference between observed and expected apoB levels based on LDL-C.
As expected, apoB levels for patients in the study with metabolic risks were higher than predicted values. However, some metabolically healthy patients also had apoB levels that varied significantly from expected measures. Physicians following U.S. guidelines may overlook people who are at higher risk of developing atherosclerosis despite normal metabolic health markers.
“I believe that our results, combined with a lot of other data showing the value of measuring apoB levels, support a revision of the guidelines to recommend apoB testing for everybody, not just those with certain clinical risk factors,” Dr. Navar said.
The study includes an online calculator for the public to estimate apoB levels based on the LDL-C level. As Dr. Navar explained, a higher-than-expected apoB level indicates a risk of heart disease that is greater than can be calculated by LDL-C alone.
Reference:
Sayed A, Peterson ED, Virani SS, Sniderman AD, Navar AM. Individual Variation in the Distribution of Apolipoprotein B Levels Across the Spectrum of LDL-C or Non–HDL-C Levels. JAMA Cardiol. 2024;9(8):741–747. doi:10.1001/jamacardio.2024.1310.
Powered by WPeMatico
Researchers have found that inclisiran significantly lowers LDL cholesterol across various glycaemic and BMI strata, with consistent efficacy and manageable safety profiles. A study was recently published in Diabetes Obesity and Metabolism journal conducted by Lawrence A. and colleagues. This pooled analysis of Phase 3 trials examined inclisiran’s performance in reducing LDL cholesterol levels in patients categorized by glycaemic status and body mass index (BMI).
Inclisiran is a novel small interfering RNA (siRNA) therapeutic that targets proprotein convertase subtilisin/kexin type 9 (PCSK9), leading to reduced LDL cholesterol levels. This study aimed to evaluate the efficacy and safety of inclisiran in diverse patient groups, stratified by glycaemic status (normoglycaemia, prediabetes, and diabetes) and BMI (<25, ≥25 to <30, ≥30 to <35, and ≥35 kg/m²).
Participants from multiple Phase 3 trials were randomized 1:1 to receive 300 mg inclisiran sodium or placebo biannually, following initial and 3-month doses for up to 18 months. All participants continued background oral lipid-lowering therapy. The primary endpoints were percentage and time-adjusted percentage changes in LDL cholesterol from baseline. The study also assessed safety across glycaemic and BMI strata.
Baseline characteristics were well-balanced between treatment groups and strata. Key findings include:
LDL Cholesterol Reduction:
• Percentage change in LDL cholesterol from baseline to Day 510 ranged from -47.6% to -51.9% across glycaemic strata and from -48.8% to -54.4% across BMI strata.
• Time-adjusted percentage changes after Day 90 up to Day 540 ranged from -46.8% to -52.0% across glycaemic strata and from -48.6% to -53.3% across BMI strata.
LDL Cholesterol Thresholds:
• The proportion of individuals achieving LDL cholesterol thresholds of <1.8 mmol/L and <1.4 mmol/L with inclisiran increased with higher glycaemic and BMI strata.
Safety:
• Mild to moderate treatment-emergent adverse events (TEAEs) at the injection site were more common with inclisiran (2.8%-7.7%) compared to placebo (0.2%-2.1%).
Inclisiran consistently reduced LDL cholesterol levels across all examined patient groups, demonstrating substantial efficacy regardless of glycaemic status or BMI. The treatment also showed a significant reduction in PCSK9 and other atherogenic lipids. These findings highlight inclisiran’s potential in managing dyslipidemia across diverse populations, offering a robust option for patients with varying metabolic profiles.
Inclisiran provides significant and sustained LDL cholesterol reduction across glycaemic and BMI strata, with a modest increase in transient mild-to-moderate injection site reactions. These results support the broader use of inclisiran in lipid management strategies, particularly for patients with higher cardiovascular risk due to diabetes or obesity.
Reference:
Leiter, L. A., Raal, F. J., Schwartz, G. G., Koenig, W., Ray, K. K., Landmesser, U., Han, J., Conde, L. G., & Wright, R. S. (2024). Inclisiran in individuals with diabetes or obesity: Post hoc pooled analyses of the ORION‐9, ORION‐10 and ORION‐11 Phase 3 randomized trials. Diabetes, Obesity & Metabolism. https://doi.org/10.1111/dom.15650
Powered by WPeMatico
