Novel shoulder arthroplasty Surgery Utilizing Groundbreaking Mixed Reality Technology unveiled in new study
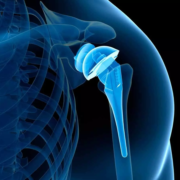
Loyola Medicine, a leader in medical innovation and technology, is proud to announce a revolutionary step forward in orthopaedic surgery. Patrick Lacey, a 49-year-old patient with a 15-year history of shoulder pain underwent a pioneering shoulder arthroplasty procedure under the skilled care of Dane Salazar, MD, a renowned shoulder and elbow specialist at Loyola Medicine.
Lacey, who underwent a right shoulder arthroscopy for bone-on-bone arthritis in 2009, was referred to Dr. Salazar because of his persistent pain and reduced range of motion. “I was so sore that I’d come home from work and put an ice pack on every day,” said Lacey. “I couldn’t put dishes in the cabinet or put on a sweatshirt without pain and cracking sounds.”
Dr. Salazar recommended a CT scan to thoroughly assess the injury, then he created a targeted treatment plan that included shoulder arthroplasty. “Dr. Salazar saw that I wanted to continue working and being active,” said Lacey. “My kids are still young and I wanted to do activities with them… like golf, softball and volleyball.”
In June 2021, Loyola made headlines by becoming one of the first hospitals in Illinois to offer this specialized shoulder replacement surgery, which is uniquely assisted by mixed reality technology. This advanced technology platform has significantly enhanced the accuracy and personalization of shoulder joint procedures. Dr. Salazar has been at the forefront of integrating mixed reality into his surgical practice. This technology allows for the overlay of 3D holographic images onto real-life settings, giving surgeons real-time access to a holographic representation of their pre-operative plan during surgery, leading to better surgical outcomes for patients like Lacey.
The mixed reality platform is beneficial during the planning and execution of surgical procedures. Patients are provided with a detailed copy of their preoperative plan, which includes both 2D and 3D representations of their shoulder anatomy and the proposed implants, leading to overwhelmingly positive feedback from patients. “We are at an exciting juncture in medical technology, where innovation meets patient care in profound ways,” said Dr. Salazar. “Utilizing the mixed reality platform allows us to take shoulder replacement surgeries to the next level of precision and personalization, offering patients a hopeful outlook for recovery and functionality.”
Loyola’s adoption of advanced medical technologies underscores its commitment to delivering state-of-the-art care and improving patient outcomes. Lacey, who underwent surgery three years ago, has made a meaningful recovery. “He’s doing fantastic,” said Dr. Salazar. “He’s back to work, with a normal range of motion and no strength deficits or pain. It’s been very rewarding for me personally to see him get back his life and to have helped improve the quality of it.”
“It’s been three years,” said Lacey. “I feel good and I have no apprehension of doing anything. I’m back to my normal self, I don’t hear the crunching sound when I’m hanging up clothes anymore. I’m back to playing softball and I can put dishes away without pain. I have full mobility, which I didn’t have for many years. I am grateful that Dr. Salazar put Humpty Dumpty back together again.”
Powered by WPeMatico