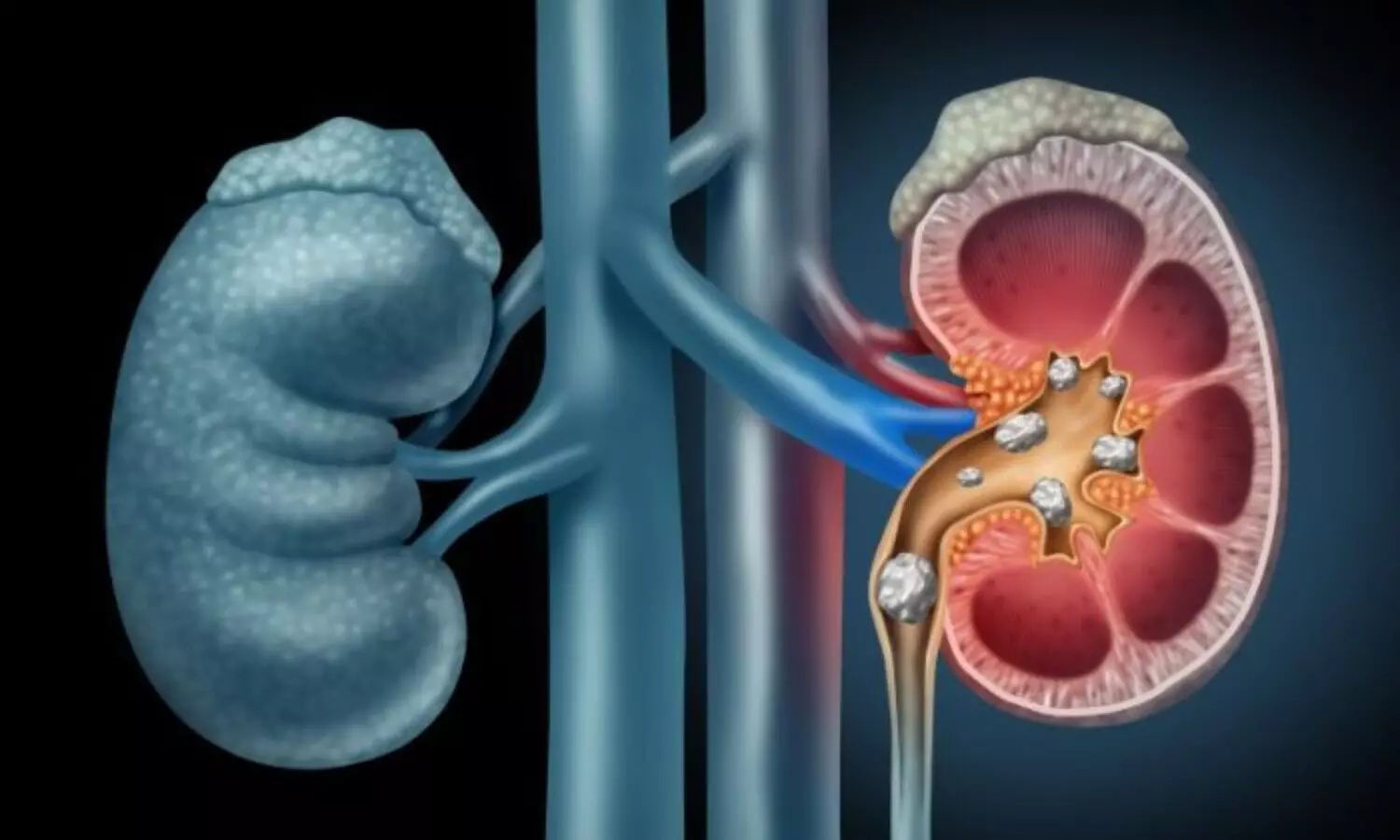
New Delhi: In a significant move to combat illegal organ transplant activities in the country, Union Minister of State for Health Smt. Anupriya Patel recently informed the Lok Sabha on the measures implemented by the Government.
As per the MoS Health, the Transplantation of Human Organs and Tissues Act, 1994 provides for an appropriate authority to be appointed by every State for investigating any complaint or breach of any of the provisions of this act, or any of the rules thereunder.
A series of questions raised by Shri Hibi Eden about the number of cases have been registered in illegal organ trade during the last five years, State/UT-wise, the conviction rate of such cases, the steps taken/proposed to be taken by the Government to curb such illegal activities, whether there are any recent regulations brought in place to prevent organ trafficking and ensure ethical organ transplantation practices.
In response to these questions, the MoS Health said, “The Transplantation of Human Organs and Tissues Act, 1994 provides for an appropriate authority to be appointed by every State for investigating any complaint or breach of any of the provisions of this act, or any of the rules thereunder. ‘Health’ and ‘Law & Order’ are State subjects. Thus, it is primarily the responsibility of the State Government/ UT Administration to take action for prevention and control of organ trafficking and monitor the same. The State Appropriate Authority shall, for the purposes of this act, have all the powers of a civil court to try a suit under the Code of Civil Procedure, 1908(5 of 1908). Whenever any complaint regarding organ trafficking is received in this Ministry, the same is referred to the concerned State/UT for appropriate action. The data in this regard is not maintained in this Ministry.”
Further, the Minister said that the National Organ and Tissue Transplant Organization (NOTTO) along with Regional Organ and Tissue Transplant Organizations (ROTTOs), State Organ and Tissue Transplant Organizations (SOTTOs) and other institutions organize awareness programs across the country to disseminate information about provisions of The Transplantation of Human Organs and Tissues Act and rules, so that people are cognizant towards the Government recognized process of organ donation permitted by the law, along with the illegality and repercussions associated with indulgence in organ trafficking, to make it easy for them to comply with the provisions of law.
Medical Dialogues team in June this year reported that the Union Government had sent out an alert to all States/Union Territories on websites and social media groups engaged in such activities after it noticed certain websites and social media posts that were promoting and offering organ trading in violation of the provisions of the Transplantation of Human Organ & Tissue Act (THOTA) 1994.
The Union Government shared the web link and other such social media posts that were being shared regularly and said that such activities were punishable offences under Section 18 of the Act with fines ranging from Rs 20 lakh to Rs 1 crore and imprisonment ranging from 5 years to 10 years.
Earlier this year, the Union Health Ministry had asked all states and UTs to investigate any violations and take appropriate action, including suspension of registration, against hospitals performing illegal organ transplants. The DGHS had urged all States and Union Territories to ensure regular collection and sharing of data on all transplant cases, including those of foreigners, with the National Organ and Tissue Transplant Organization (NOTTO) every month.
The Ministry had issued the direction around two weeks after an organ trafficking racket, involving Bangladeshi nationals, being run in Haryana and Rajasthan was busted.
The following steps taken by the Govenment to curb illegal organ transplant activities, as informed by MoS Health are as follows:
• A letter was sent by the Ministry of Health and Family Welfare to the Ministry of External Affairs (MEA) after which, a note verbale was circulated by MEA to all the Embassies/ Foreign Missions in India apprising them about the legal provisions of the organ transplant Act in India to prevent illegal organ transplants involving foreigners. Rules for transplants involving foreigners were shared with the Ministry of External Affairs for dissemination to Indian Missions abroad and the same have also been displayed on ports and Airports.
• States/UTs have been requested to ensure that every hospital performing organ transplantation or retrieval needs to be linked to the website of the National Organ & Tissue Transplant Organization (NOTTO) and data related to both deceased and living donors and recipients of transplants is required to be uploaded in the National Registry maintained by NOTTO. Further, each donor and recipient of human organ will have a unique NOTTO ID in cases of both deceased as well as living donor transplant and the same is to be generated by the concerned hospitals.
•All States/UTs have been advised to constitute an Advisory Committee as per provisions of the Transplantation of Human Organs and Tissues Act (THOTA), 1994 to aid and advise the Appropriate Authority in discharging its functions of controlling illegal organ transplant activities.
Also read- Centre Sends Alerts To States, UTs On Websites, Social Media Involved In Illegal Trading Of Human Organs
In another set of questions raised by Dr Bhola Singh regarding the government fixing any timeline for organ transplantation from living donors, the Minister said that the health department has prescribed timelines for the functioning of the Authorization Committee constituted under the Transplantation of Human Organs and Tissues Act (THOTA), 1994 for the guidance of State / Union Territory to expedite the decision-making process regarding living donor organ transplantation.
Further, Dr Singh asked about the steps taken/proposed to be taken by the Government to ensure compliance with this timeline under the Transplantation of Human Organs and Tissues Act, 1994, whether a dedicated task force or committee is proposed to be established to monitor adherence to these timelines, the measures being taken to address delays in organ transplantation, specifically in cases where patients have died waiting for permissions; and whether any amendments are proposed to The Transplantation of Human Organs and Tissues Act, 1994 to improve the process and if so, the details thereof along with the other steps taken/proposed to be taken in this regard.
In response to these questions, the Minister said, “It is for the State Government / UT Administration to implement and monitor while ensuring compliance with the aforesaid timelines. THOTA provides for an Appropriate Authority in every State to be appointed by the State Government which is authorized to undertake such measures as may be prescribed. State Government is also authorized to constitute by notification the Authorization Committee at the State / district / hospital level. THOTA also provides for an Advisory Committee to be constituted by the State by notification to aid and advise the Appropriate Authority in discharging its functions.”
To review the THOTA, 1994, a ‘National symposium on Legal and Ethical Aspects of Organ Donation and Transplantation’ involving State/UT representatives, organ donation and transplantation experts, legal experts, NGOs and other relevant stakeholders was organised in January,2024 by National Organ & Tissue Transplant Organisation (NOTTO), the minister further stated.
Last year, the Medical Dialogues team reported that India had achieved more than 15,000 transplants in a year (2022) for the first time. Along with this, there was an annual increase of 27% in transplant numbers. This was informed by Union Health Secretary Shri Rajesh Bhushan at the National Organ & Tissue Transplant Organisation (NOTTO) Scientific Dialogue 2023.
The dialogue was organized to bring all the stakeholders under one roof to brainstorm ideas about interventions and best practices in the organ and tissue transplant field that can be taken up to save lives.
Also read- Over 15000 Organ Transplants Recorded In 2022, Union Health Secretary Says At NOTTO Scientific Dialogue 2023•