Could Rejuvenating Mitochondria Help Fight Alzheimer’s? Study sheds light

Powered by WPeMatico

Powered by WPeMatico
Novartis today presented results from the 6-month, double-blind period of the Phase III APPEAR-C3G study of Fabhalta (iptacopan) at the late-breaking clinical trials session of the European Renal Association (ERA) Congress. Patients treated with Fabhalta in addition to supportive care achieved a 35.1% (p=0.0014) reduction in proteinuria (as measured by 24-hour urine protein to creatinine ratio [UPCR]) at 6 months when compared to placebo on top of supportive care. In many kidney diseases, proteinuria reduction is an increasingly recognized surrogate marker correlating with delaying progression to kidney failure.
Fabhalta is an oral Factor B inhibitor of the alternative complement pathway being investigated in adult patients with C3 glomerulopathy (C3G). Regulatory submissions, including to the FDA and EMA, for the adult C3G indication are planned for the second half of 2024.
“C3G is an overlooked and devastating illness that often strikes when people are young. The prognosis for patients with C3G is poor, and around half of the affected patients progress to kidney failure requiring dialysis or transplant within 10 years of being diagnosed,” said Marianne Silkjær Nielsen, Founder of CompCure, a Danish non-profit association committed to improving outcomes for individuals with C3G and immune complex membranoproliferative glomerulonephritis (IC-MPGN). “Currently there are no therapies approved for C3G, but research into potential new treatments developed specifically for this disease gives us hope that we can improve outcomes for patients and blunt its emotional, physical and social effects.”
Additional data on the secondary endpoint of estimated glomerular filtration rate (eGFR), a measure of kidney function, showed a numerical improvement of +2.2 mL/min/1.73 m2 (p=0.1945) over 6 months with Fabhalta compared to placebo. The study also showed Fabhalta has a favorable safety profile with no new safety signals.
“This is an exciting milestone for patients and the potential future management of C3G. The hallmark of C3G is overactivation of part of the immune system called the alternative complement pathway, which damages the kidneys and leads to severe loss of kidney function in many patients. Currently used treatments don’t address the underlying biology of C3G and often come with significant side effects that add to the burden of the illness,” said Professor David Kavanagh, Professor of Complement Therapeutics & Honorary Consultant Nephrologist at the Faculty of Medical Sciences at Newcastle University and APPEAR-C3G Steering Committee Member. “Fabhalta is the first potential treatment that targets the alternative complement pathway in C3G, and its impact on measures of kidney damage and kidney function in this study, in addition to its safety profile, is encouraging for patients and the clinical community.”
The APPEAR-C3G study continues with an additional 6-month, open-label period following the 6-month double-blind period, in which all patients receive Fabhalta, including those previously receiving placebo, These data will be presented at an upcoming medical meeting when available.
At ERA, Novartis is also presenting new data across its rare disease portfolio, including results for investigational atrasentan in IgA nephropathy (IgAN) from the 36-week interim analysis of the Phase III ALIGN study, additional data for Fabhalta in IgAN from the 9-month interim analysis of the Phase III APPLAUSE-IgAN study, long-term 33-month efficacy and safety data for Fabhalta in C3G from the Phase II extension study, 1-year Phase I/II data for investigational zigakibart in IgAN, and data from real-world studies in C3G and atypical hemolytic uremic syndrome (aHUS).
“Our ambition is to transform the care of patients living with rare kidney diseases by discovering, developing and delivering innovative treatment options,” said David Soergel, M.D., Global Head, Cardiovascular, Renal and Metabolism Development Unit, Novartis. “The APPEAR-C3G results add to the growing body of evidence demonstrating Fabhalta’s potential to target the underlying pathophysiological drivers and to provide clinically meaningful outcomes in a range of rare conditions.”
APPEAR-C3G (NCT04817618) is a Phase III multicenter, randomized, double-blind, parallel group, placebo-controlled study to evaluate the efficacy and safety of twice-daily oral Fabhalta (200 mg) in C3G patients. In addition to the results from adult patients with C3G, enrollment is ongoing in a separate cohort of adolescent patients with C3G2,3. The study comprises a 6-month double-blind period where adult patients were randomized 1:1 to receive Fabhalta or placebo on top of supportive care, followed by a 6-month open-label period where all patients receive Fabhalta (including those who were previously on placebo).
The primary endpoint for the double-blind period was proteinuria reduction from baseline at 6 months for Fabhalta compared to placebo as measured by 24-hour UPCR. The primary endpoint for the open-label period is proteinuria reduction from baseline at 12 months for both treatment arms and proteinuria reduction from 6 to 12 months for the placebo arm. Secondary endpoints for the double-blind period include change in eGFR, proportion of participants meeting composite renal endpoint criteria (≤15% reduction in eGFR and ≥50% reduction in UPCR), change in glomerular inflammation (as measured by disease total activity score in renal biopsy), change in patient reported fatigue (as measured by FACIT-Fatigue score), and safety and tolerability.
Fabhalta (iptacopan) is an oral, Factor B inhibitor of the alternative complement pathway.
Discovered at Novartis, Fabhalta is currently in development for a range of rare diseases including IgAN, C3G, aHUS, IC-MPGN and lupus nephritis (LN), and, as such, the safety and efficacy profile have not been established in these indications. There is no guarantee that Fabhalta will become commercially available for these indications.
Fabhalta was approved by the FDA in December 2023 and the EMA in May 2024 for the treatment of adults with the rare blood disorder paroxysmal nocturnal hemoglobinuria (PNH).
C3G is an ultra-rare, progressive kidney disease that initially presents in mostly children and young adults. Each year, approximately 1-2 people per million worldwide are newly diagnosed with C3G, a form of membranoproliferative glomerulonephritis (MPGN).
In C3G, overactivation of the alternative complement pathway – part of the immune system – causes deposits of C3 protein to build up in kidney glomeruli (a network of blood vessels that filter waste and remove extra fluids from the blood). This triggers inflammation and glomerular damage that results in proteinuria (protein in urine), hematuria (blood in urine) and reduced kidney function. Approximately 50% of C3G patients progress to kidney failure within 10 years of diagnosis, at which point they will require dialysis and/or kidney transplantation6,7, with over 55% of patients with C3G experiencing disease recurrence post-transplant.
Powered by WPeMatico

A recent research published in the Journal of Inflammation Research uncovered a potential link between low levels of vitamin K and increased risk of depression and suicidal behavior. Despite the limited epidemiological studies in this area, this new study highlighted strong evidence which suggests vitamin K deficiency could be a significant biological risk factor for these mental health issues.
This retrospective cross-sectional analysis involved a total of 295 participants and divided into two groups, where 146 individuals had a history of suicide attempts and 149 individuals had no history of suicide attempts. This research measured various biological markers, including thyroid hormones, lipid profiles, inflammatory cytokines and vitamins to explore the potential associations with depression and suicidal behavior.
The results found that the participants who had attempted suicide expressed markedly lower levels of FT4 (free thyroxine), total cholesterol (TC), vitamin D and vitamin K. Also, these individuals showed increased levels of C-reactive protein (CRP) which indicates inflammation.
Among the different variables examined, vitamin K emerged as a strong predictor of suicidal behavior in depressed patients. The research reported a sensitivity of 0.842 and a specificity of 0.715 for vitamin K levels in diagnosing the suicidal attempts among the depressed individuals. This means that vitamin K levels were accurate in identifying the individuals at risk for suicidal behavior in over 80% of cases.
Correlation analysis further revealed that vitamin K levels were significantly and positively related to several other biomarkers, including FT4, total cholesterol, LDL cholesterol and small dense LDL cholesterol. These findings suggest a broader interaction between vitamin K and other physiological processes that may influence mental health.
The multivariate analysis illuminated the importance of vitamin K as a predictive factor for suicidal attempts in patients with depression. Also, the study found that serum vitamin K levels significantly predicted suicidal behavior (Odds Ratio = 0.614, P = 0.004, 95% Confidence Interval 0.153–0.904). The outcomes observed a negative correlation between vitamin K levels and suicidal attempts when analyzing the data for FT4, CRP and vitamin D. This indicates that lower levels of vitamin K are associated with a higher risk of suicide attempts in the context of other health markers.
The findings of this study suggest that monitoring and managing vitamin K levels could play a crucial role in the prevention and treatment of depression and suicidal behavior. Given the significant diagnostic value of vitamin K, clinicians might consider incorporating vitamin K assessments into routine evaluations for patients at risk of depression and suicidal tendencies.
Reference:
Wang, S.-T., He, X.-Y., Le, J., Sun, T., & Peng, R. (2024). Associations Between Vitamin K and Suicide Attempts in Patients with Depression: A Case-Control Study. In Journal of Inflammation Research: Vol. Volume 17 (pp. 3423–3431). Informa UK Limited. https://doi.org/10.2147/jir.s463204
Powered by WPeMatico

Researchers from Fred Hutch Cancer Center have found that active surveillance for prostate cancer patients with a low risk of progression is an effective alternative to immediate surgery or radiation to manage the disease. They found that half of men with low-risk prostate cancer remained free from progression or treatment 10 years after diagnosis when followed in a protocol-directed active surveillance program. This finding highlights the viability of active surveillance as a management strategy for favorable-risk prostate cancer, alleviating concerns about delayed treatment leading to worse outcomes. The results of the study have been published in JAMA .
Active surveillance has emerged as the preferred management strategy for low-grade prostate cancer, involving regular PSA exams and prostate biopsies to monitor disease progression. Despite this, only about 60% of eligible patients opt for surveillance due to fears of undertreatment and missing a window of curability. Current clinical guidelines offer limited direction on the optimal surveillance approach, necessitating further research.
The study analyzed data from the Canary Prostate Active Surveillance Study (PASS), a collaborative observational study involving 10 North American centers. Participants included 2,155 men with favorable-risk prostate cancer, enrolled from 2008 through 2022. The median age was 63, and the median follow-up period was 7.2 years. The primary endpoints were biopsy grade reclassification, treatment, metastasis, prostate cancer mortality, overall mortality, and recurrence after treatment. The key findings were as follows
• 50% of men remained free from progression or treatment at 10 years.
• 43% had biopsy grade reclassification.
• 49% underwent treatment for prostate cancer.
• 18% required treatment after confirmatory biopsy (median 1.5 years).
• 18% required treatment after subsequent biopsies (median 4.6 years).
• 5-year recurrence rate: 11% (early treatment), 8% (later treatment).
• 10-year metastasis rate: 1.4%.
• Prostate cancer-specific mortality at 10 years: 0.1%.
• Overall mortality at 10 years: 5.1%.
The study’s findings support the safety and effectiveness of active surveillance for managing low-risk prostate cancer. High adherence to biopsy schedules likely contributed to the low rates of metastasis and recurrence. Notably, the Canary PASS cohort had an 88% adherence to first follow-up biopsies within 2 years of diagnosis and 97% within 5 years. These results align with the premise that regular monitoring during surveillance is a safe management strategy.
Active surveillance, involving regular PSA exams and prostate biopsies, is a safe and effective management strategy for favorable-risk prostate cancer. The study’s findings suggest that delayed treatment does not lead to worse outcomes compared to earlier treatment, reinforcing the potential of active surveillance to become a widely accepted approach for managing low-risk prostate cancer.
Reference:
Newcomb, L. F., Schenk, J. M., Zheng, Y., Liu, M., Zhu, K., Brooks, J. D., Carroll, P. R., Dash, A., de la Calle, C. M., Ellis, W. J., Filson, C. P., Gleave, M. E., Liss, M. A., Martin, F., McKenney, J. K., Morgan, T. M., Tretiakova, M. S., Wagner, A. A., Nelson, P. S., & Lin, D. W. (2024). Long-term outcomes in patients using protocol-directed active surveillance for prostate cancer. JAMA: The Journal of the American Medical Association. https://doi.org/10.1001/jama.2024.6695
Powered by WPeMatico

Canada: A leading biopharmaceutical company dedicated to innovative cannabinoid-based therapies, Avicanna Inc., has recently unveiled the results of a groundbreaking study focused on patients suffering from Epidermolysis Bullosa (EB). This debilitating genetic disorder, characterized by fragile skin prone to blistering and tearing, has long posed significant challenges for both patients and healthcare providers. However, Avicanna’s latest research offers a ray of hope, showcasing the potential of cannabinoid-based treatments in managing EB symptoms and improving patients’ quality of life.
The company announced the completion of the retrospective observational real-world evidence study (“Study”) using its RHO Phyto branded Ultra CBD Topical Cream on patients with epidermolysis bullosa.
The study, led by Elena Pope, MD, M.Sc., FRCPC, Head of Dermatology at The Hospital for Sick Children in Toronto, evaluated the efficacy and tolerability of RHO Phyto branded Ultra CBD Topical Cream in patients with epidermolysis bullosa.
The retrospective cohort study evaluated the reported and documented responses related to wound healing, itch, and pain endpoints through images for study purposes to evaluate and examine RHO Phyto branded Ultra CBD Topical Cream’s effect on wound healing. The RHO Phyto branded Ultra CBD Topical Cream is an oil-based 3% CBD localized cream developed to target dermatology conditions.
The study involved 20 patients (6 female patients and 14 male patients) with an average age of 17.3 years with various subtypes of epidermolysis bullosa, including simplex (30%), dystrophic (60%), and junctional (10%).
The following were the key findings of the study:
The study results will be presented at Avicanna’s symposium on May 13th by Dr. Camila Sofia Arriaga Egnen from The Hospital for Sick Children.
Dr. Elena Pope stated, “Using RHO Phyto branded Ultra CBD Topical Cream is a novel topical therapeutic option for EB patients, providing symptom relief and potentially aiding in wound healing with good tolerability. Further prospective studies are needed to substantiate these findings.”
“We are pleased to see the Study reporting early positive results for RHO Phyto branded Ultra CBD Topical Cream in a patient population that continues to seek treatment for its catastrophic condition,” stated Karolina Urban, PhD, Executive Vice President of Medical Affairs, Avicanna Inc. Dr. Urban further stated,
“These results are critical in helping guide us in the next steps in the further development of our medical products and pharmaceutical pipeline.”
Powered by WPeMatico
For patients with chronic migraine, nerve decompression surgery effectively reduces the number of headache days-the outcome measure preferred by neurologists-along with other measures including the frequency and intensity of migraine attacks, reports a study in the June issue of Plastic and Reconstructive Surgery®, the official medical journal of the American Society of Plastic Surgeons (ASPS). The journal is published in the Lippincott portfolio by Wolters Kluwer.
“Neurologists evaluating migraine treatments tend to focus on reduction in headache days, while plastic surgeons performing headache surgery are more likely to use a measure incorporating other headache outcomes, such as the Migraine Headache Index,” comments ASPS Member Surgeon and Professor of Plastic Surgery, Surgery, Neurosurgery and Neurology Jeffrey E. Janis, MD, of The Ohio State University Wexner Medical Center, Columbus. “Our study adds new evidence that headache surgery improves both sets of measures, providing a more comprehensive assessment of the results of headache surgery.”
Peripheral nerve decompression surgery-sometimes called trigger point deactivation or headache surgery – has become an established surgical treatment for chronic migraine and certain other neurological causes of headache, such as occipital and supraorbital neuralgia. Migraine surgery seeks to relieve nerve compression at trigger sites in the head and neck, which are thought to contribute to headaches.
When plastic surgeons evaluate the outcomes of headache surgery, they typically use the Migraine Headache Index (MHI), which incorporates the frequency, intensity, and duration of migraine attacks. In contrast, neurologists-“the traditional experts on nonsurgical migraine treatment”-focus on the change in monthly migraine days.
“This discrepancy is one reason why some headache specialists have been slow to recognize the growing body of evidence showing the effectiveness of headache surgery,” says Dr. Janis. Current guidelines recommend against evaluating headache intensity or duration, citing a lack of standardization.
To help bridge the gap between specialties, the researchers reviewed 19 studies of headache surgery that reported information on monthly migraine days. Performed between 2005 and 2020, the studies included a total of 1,603 patients. Five of the studies were randomized controlled trials, the highest level of research evidence.
Of eight studies assessing monthly migraine days before and after migraine surgery, six showed a significant reduction in days with migraine attacks. On weighted analysis, patients averaged 14.11 fewer migraine days per month, from before to after surgery. Based on 12 studies, total migraine attacks decreased by 8.65 days per month.
Other outcomes also improved after headache surgery, including an average reduction of 76.59 points (out of a maximum of 300 points) in total MHI score. This included improvements in migraine intensity, which decreased by an average of 3.84 points (on a 0-to-10 scale); and attack duration, which decreased by 11.80 hours per month. The studies reported no major complications of headache surgery.
The study “demonstrates the efficacy of headache surgery on the outcomes used in both the [plastic surgery] and neurology literature,” Dr. Janis and coauthors conclude. They acknowledge some limitations of their study – notably including the variability in the trigger sites addressed by headache surgery. Nevertheless, the findings “provide strong evidence in support of headache surgery efficacy.”
“We hope our study will help to foster common communication between plastic surgeons and neurologists in assessing the effects of headache surgery for patients with chronic headache pain,” Dr. Janis comments. “Future studies of headache surgery should routinely include data on monthly migraine days, in order to better compare the outcomes of surgical and medical treatments.”
Reference:
Ormseth BH, ElHawary H, Huayllani MT, Weber KD, Blake P, Janis JE. Comparing Migraine Headache Index versus Monthly Migraine Days after Headache Surgery: A Systematic Review and Meta-Analysis. Plast Reconstr Surg. 2024 Jun 1;153(6):1201e-1211e. doi: 10.1097/PRS.0000000000010800.
Powered by WPeMatico

A recent study published in Thorax Journal highlighted the significant impact of social media on the health behaviors of children and young people, particularly regarding the cigarette and e-cigarette use. The research was conducted using data from the UK Household Longitudinal Study from 2015 to 2021 and uncovered concerning correlations between the amount of time spent on social media and the smoking risk among youth aged 10 to 25 years.
The study analyzed responses from a total of 10,808 participants and encompassed close to 27,962 observations over the six-year period. The study investigated the relationship between the amount of social media use on a typical weekday and current smoking habits, including both traditional cigarettes and e-cigarettes. The analysis employed generalized estimating equation (GEE) logistic regression models to determine these associations after adjusting for a range of potential confounders such as age, sex, UK region, ethnicity, household income and the smoking behaviors of others in the household.
The study found that 8.6% of the participants reported smoking cigarettes at some point during the study, while 2.5% reported using e-cigarettes. The study revealed a clear association between higher social media use and increased odds of smoking. Also, the participants who spent seven or more hours per day on social media had a significantly higher likelihood of smoking cigarettes, with an adjusted odds ratio (AOR) of 3.60 (95% CI 2.61 to 4.96) when compared to the participants who did not use social media.
Similar trends were observed for the use of e-cigarette. The odds of using e-cigarettes were significantly higher among heavy social media users (AOR 2.73, 95% CI 1.40 to 5.29 for ≥7 hours/day vs none). The study found strong evidence of a dose-response relationship by indicating that the risk of smoking increased with the amount of time spent on social media (both p<0.001).
When the data were broken down by sex and household income, the associations remained consistent for cigarette smoking across all groups which found more pronounced e-cigarette use among the individuals from higher-income households. This study highlights the potential public health risks associated with excessive social media use among young people. The clear association between social media exposure and increased smoking behaviors suggests a need for increased awareness and further research.
Source:
Hopkinson, N. S., Vrinten, C., Parnham, J. C., Radó, M. K., Filippidis, F., Vamos, E. P., & Laverty, A. A. (2024). Association of time spent on social media with youth cigarette smoking and e-cigarette use in the UK: a national longitudinal study. In Thorax (p. thorax-2023-220569). BMJ. https://doi.org/10.1136/thorax-2023-220569
Powered by WPeMatico

China: In a groundbreaking development, a recent bidirectional Mendelian randomization study has uncovered a compelling causal association between low vitamin D levels and polycystic ovary syndrome (PCOS) incidence. This finding sheds new light on the intricate interplay between hormonal imbalances and nutritional factors in the development of this common endocrine disorder affecting women of reproductive age.
The findings, published in the Journal of Ovarian Research revealed that genetically predicted lower serum vitamin D (VD) levels may cause a higher risk of developing PCOS, which may be mediated by increased production of testosterone.
PCOS is characterized by a combination of symptoms, including irregular menstrual cycles, excess androgen levels, and ovarian cysts. While its exact etiology remains elusive, previous research has implicated genetic predispositions and environmental influences. Among these factors, vitamin D deficiency has long been suspected to play a role, given its regulatory functions in reproductive health and immune system modulation.
Bingrui Gao, The First Affiliated Hospital of China Medical University, Shenyang, Liaoning, P.R. China, and colleagues aimed to investigate the cause-effect relationship between serum VD and PCOS, and the role of testosterone in the related pathological mechanisms.
For this purpose, the research team assessed the causality between serum VD and PCOS by using genome-wide association studies (GWAS) data in a bidirectional two-sample Mendelian randomization (TS-MR) analysis.
Subsequently, they conducted an MR mediation analysis to examine the mediating action of testosterone in the causality between serum VD and PCOS. Ultimately, they integrated GWAS data with cis-expression quantitative loci (cis-eQTLs) data for gene annotation, and potentially related genes were used for functional enrichment analysis to evaluate the involvement of testosterone and the potential mechanisms.
Based on the study, the researchers reported the following findings:
• TS-MR analysis showed that individuals with lower serum VD levels were more likely to develop PCOS (OR = 0.750).
• MR mediation analysis uncovered an indirect causal effect of serum VD level on the risk of PCOS via testosterone (OR = 0.983).
• Functional enrichment analysis showed that several pathways may be involved in the VD-testosterone-PCOS axis, such as steroid hormone biosynthesis and autophagy process.
“Our studies confirmed the causality between lower serum vitamin D level and higher PCOS risk,” the researchers wrote. “Furthermore, testosterone may act as a mediator between serum VD and PCOS.”
“These findings emphasize the clinical importance of testing serum vitamin D level and timely VD supplementation as possible primary prevention and treatment of PCOS,” they concluded.
Reference:
Gao, Bingrui, et al. “Causal Association Between Low Vitamin D and Polycystic Ovary Syndrome: a Bidirectional Mendelian Randomization Study.” Journal of Ovarian Research, vol. 17, no. 1, 2024, p. 95.
Powered by WPeMatico
Researchers have found that intermittent intravenous (IV) paracetamol significantly reduces morphine consumption in young children undergoing cardiac surgery with cardiopulmonary bypass. This multicenter, randomized, double-blind, controlled trial conducted in Pediatric Intensive Care Units (PICUs) across the Netherlands and Belgium, assessed the effectiveness of IV paracetamol as the primary analgesic option compared to continuous morphine use. The study was published in the journal Critical Care by Gerdien Zeilmaker-Roest and colleagues.
Morphine has traditionally been used for pain management in children undergoing cardiac surgery, but it comes with risks such as respiratory depression. Paracetamol is a potential alternative to reduce these risks while still providing effective pain management.
The study included 194 patients aged 0-3 years who underwent cardiac surgery with cardiopulmonary bypass between March 2016 and July 2020. Patients were randomized to either continuous morphine or intermittent IV paracetamol as the primary analgesic after receiving a loading dose of 100 mcg/kg of morphine at the end of surgery. Rescue morphine was administered if numeric rating scale (NRS) pain scores exceeded a predetermined threshold.
The primary outcome was the median weight-adjusted cumulative morphine dose (mcg/kg) in the first 48 hours post-surgery. The study used the nonparametric Van Elteren test, stratified by center, for primary outcome comparison.
The key findings of the study were:
The IV paracetamol group had a median weight-adjusted cumulative morphine dose of 145.0 mcg/kg (IQR 115.0-432.5 mcg/kg) compared to 692.6 mcg/kg (IQR 532.7-856.1 mcg/kg) in the continuous morphine group.
This represents a 79% reduction in morphine use (P < 0.001).
Both groups showed similar rescue morphine consumption (P = 0.38) and similar levels of pain relief, suggesting non-inferiority of IV paracetamol administration in terms of NRS pain scores.
The non-inferiority analysis revealed a difference in proportion of patients with NRS pain scores of 4 or higher between the groups at -3.1% (95% CI -16.6-10.3%).
These findings suggest that IV paracetamol is an effective primary analgesic option for children aged 0-3 years post-cardiac surgery, offering significant reductions in morphine use without compromising pain relief. This could be a safer and potentially more efficient approach to post-surgical pain management.
The use of intermittent IV paracetamol as the primary analgesic in children aged 0-3 years after cardiac surgery with cardiopulmonary bypass results in a significant 79% reduction in median weight-adjusted cumulative morphine consumption over the first 48 hours post-surgery. The findings suggest that IV paracetamol provides equal pain relief compared to continuous morphine, indicating its potential as a safer and effective primary analgesic option for young children post-cardiac surgery.
Reference:
Zeilmaker-Roest, G., de Vries-Rink, C., van Rosmalen, J., van Dijk, M., de Wildt, S. N., Knibbe, C. A. J., Koomen, E., Jansen, N. J. G., Kneyber, M. C. J., Maebe, S., Van den Berghe, G., Haghedooren, R., Vlasselaers, D., Bogers, A. J. J. C., Tibboel, D., & Wildschut, E. D. (2024). Intermittent intravenous paracetamol versus continuous morphine in infants undergoing cardiothoracic surgery: a multi-center randomized controlled trial. Critical Care (London, England), 28(1). https://doi.org/10.1186/s13054-024-04905-3
Powered by WPeMatico
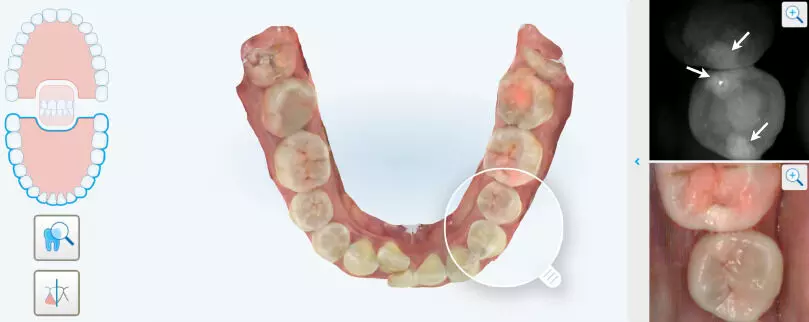
NIRI promising radiation-free method for early detection of proximal caries in permanent teeth suggests a study published in the Journal of Dentistry.
This study aimed to evaluate the diagnostic performance of near-infrared imaging (NIRI) and unaided visual examination (UVE) in detecting proximal caries in permanent dentition in comparison with cone-beam computed tomography (CBCT). Patients who underwent NIRI, UVE, and CBCT imaging within 1 week were enrolled. Using CBCT as the reference test, the positive percent agreement (PPA), negative percent agreement (NPA), and overall percent agreement (OPA) of NIRI, UVE, and a combination of the two for detecting proximal caries at different depths and in different tooth locations were assessed. Additionally, the consistency of these diagnostic methods with CBCT was evaluated. Results: They evaluated 6,084 proximal surfaces and identified 177 CBCT-positive sites. NIRI had a PPA, NPA, and OPA of 68.93 %, 99.09 %, and 98.21 %, respectively, with a substantial agreement with CBCT. When combined with UVE, the PPA increased by approximately 50 % compared with that of UVE alone. Regarding caries at different depths, NIRI outperformed UVE in detecting initial caries (ICDAS 1–2) over moderate-to-advanced caries (ICDAS 3–6). However, the combined use of NIRI and UVE improved the detection of moderate-to-advanced caries. In the anterior teeth region, NIRI exhibited excellent agreement with CBCT, surpassing its performance in the posterior region. Although NIRI cannot fully replace radiographic methods, the substantial agreement of NIRI with CBCT in detecting proximal caries highlights its potential as a complementary tool in routine caries screening, especially when combined with UVE. This study highlights the potential of NIRI as a radiation-free method for detecting proximal caries in permanent teeth. Early detection through regular NIRI scanning can lead to timely intervention, improved patient outcomes, and reduced overall disease burden.
Reference:
Kai Xia, Wenxin Lu, Zhongcheng Li, Yang Zhang, Rui Ye, Zhihe Zhao,
Comparison of near-infrared imaging with cone-beam computed tomography for proximal caries detection in permanent dentition: An in vivo study. Journal of Dentistry, Volume 145,
2024, 104994, ISSN 0300-5712. https://doi.org/10.1016/j.jdent.2024.104994.
(https://www.sciencedirect.com/science/article/pii/S0300571224001647)
Keywords:
NIRI, promising, radiation-free, method, early, detection, proximal caries, permanent teeth, Study, Journal of Dentistry, Kai Xia, Wenxin Lu, Zhongcheng Li, Yang Zhang, Rui Ye, Zhihe Zhao, Near-infrared light; Intraoral scan; CBCT; Dental caries diagnosis; Proximal caries
Powered by WPeMatico
