People with well-controlled, long-duration type 1 diabetes may still face high risk of heart disease
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico

Tamil Nadu- Similar to the National Institute of Mental Health and Neurosciences in Bengaluru, Tamil Nadu is now set to establish the Institute of Mental Health and Neurosciences (TN-IMHNS) by early 2025. In March 2023, Rs 35 crore was sanctioned for TN-IMHNS’s new building.
As per the recent report by TOI, the state government in the held assembly session announced that it will set up a centre to provide comprehensive care for neurology and psychiatry under one roof.
Meanwhile, talking about the construction of TN-IMHNS, M Malayappan, director of the Institute of Mental Health told TOI, “Construction began in Aug 2023, and three of the six floors have been completed. It will take another eight to 10 months to complete it”.
TN-IMHNS will be a centre of excellence that will conduct postgraduate and doctoral programmes in mental health and neurosciences and will also provide training programmes for mental health professionals. It will also be equipped to conduct research on a wide range of mental health and neurological disorders.
The institute will provide mental health services for people of all ages with a team of highly qualified psychiatrists, psychologists, psychiatric social workers, nurses and physicians. Though this centre does not provide treatment for problems such as speech disorders or movement disorders that people suffer after stroke or brain surgery, the new centre will be able to incorporate treatment and rehabilitation for such people as well, said M Malayappan, director of the Institute of Mental Health.
Powered by WPeMatico

Paris: Sanofi has announced the completion of its acquisition of Inhibrx, Inc. The acquisition adds SAR447537 (formerly INBRX-101) to Sanofi’s rare disease pipeline.
SAR447537 is a human recombinant protein that holds the promise of allowing alpha-1 antitrypsin deficiency (AATD) patients to achieve normalization of serum AAT levels with less frequent (monthly vs. weekly) dosing. AATD is an inherited rare disease characterized by low levels of AAT protein, predominantly affecting the lung with progressive deterioration of the tissue. SAR447537 may help to reduce inflammation and prevent further deterioration of lung function in affected individuals.
The former holders of shares of Inhibrx common stock voted to approve the acquisition at a special meeting of stockholders on May 24, 2024. Upon the closing of the acquisition, former shareholders of Inhibrx became entitled to receive $30.00 per share in cash, which represents a total equity value of approximately $1.7 billion (on a fully diluted basis), as well as one contingent value right per share to receive $5.00 upon the achievement of a regulatory milestone.
Sanofi completed its acquisition of Inhibrx through the merger of an indirect, wholly owned subsidiary of Sanofi with and into Inhibrx, with Inhibrx continuing as the surviving corporation and becoming an indirect, wholly owned subsidiary of Sanofi.
Prior to the closing of the acquisition, Inhibrx completed the spin-off of Inhibrx Biosciences, Inc., distributing 92% of Inhibrx Biosciences’s shares to holders of shares of Inhibrx common stock as of May 17, 2024. Inhibrx Biosciences, which was a wholly owned subsidiary of Inhibrx prior to the distribution, acquired all of the assets of Inhibrx not related to SAR447537, which include INBRX-109 and INBRX-1061, as well as all Inhibrx employees, pursuant to an internal reorganization. Inhibrx continues to own the remaining 8% of Inhibrix Biosciences following the completion of the transactions. Inhibrx Biosciences began trading on the NASDAQ Global Market on May 30, 2024, under the ticker “INXB” and, beginning on May 31, 2024, will trade under the ticker “INBX”.
As of May 30, 2024, Inhibrx common stock will cease to be traded on the NASDAQ Global Market and will be subsequently deregistered.
Lazard acted as exclusive financial advisor to Sanofi and Weil, Gotshal & Manges LLP acted as its legal counsel. Centerview Partners LLC acted as exclusive financial advisor to Inhibrx and Paul, Weiss, Rifkind, Wharton and Garrison LLP served as legal counsel.
Read also: USFDA requests additional data on Sanofi Dupixent for treating smoker’s lung
Powered by WPeMatico
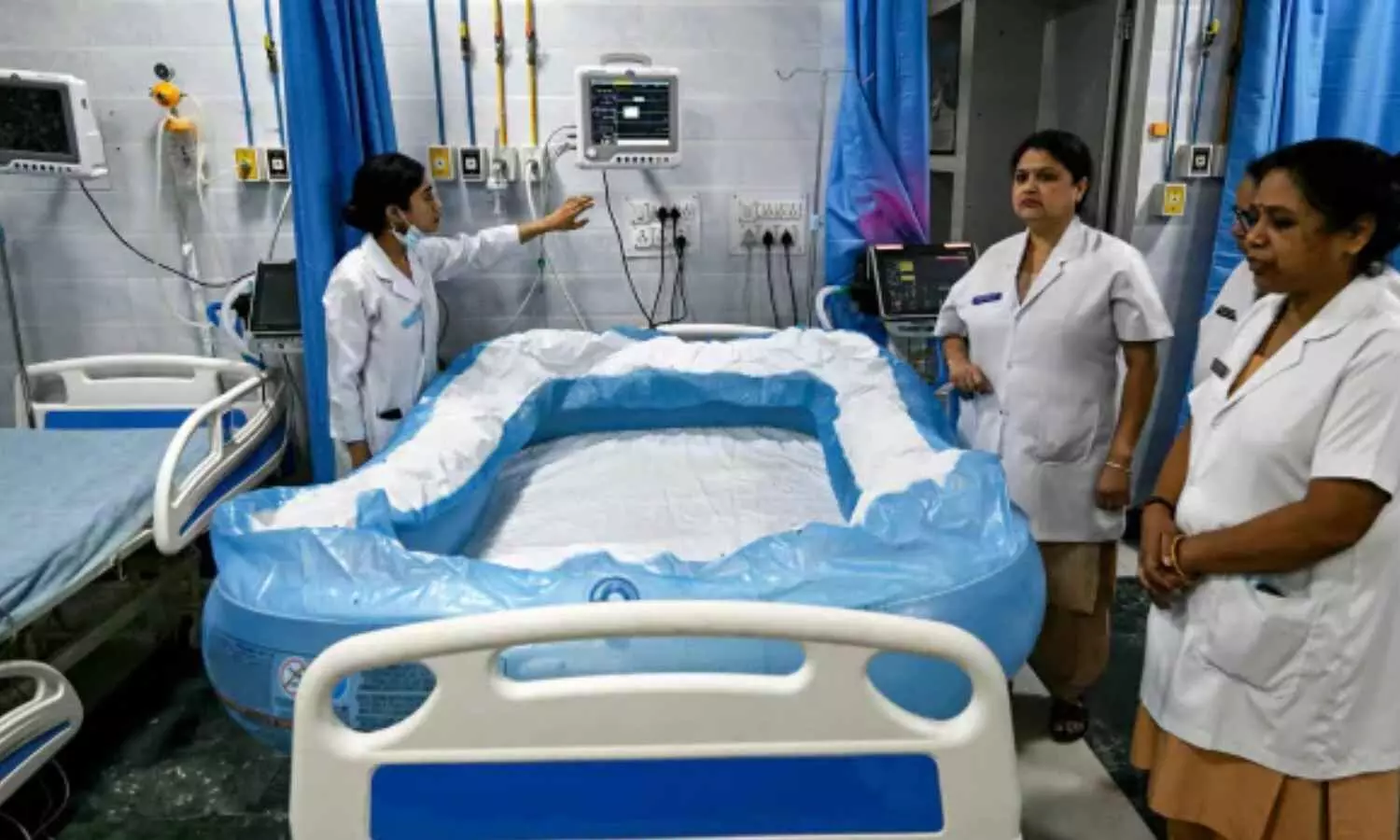
New Delhi: In response to the rising temperatures and heat waves in the capital, Ram Manohar Lohia (RML) Hospital has made special arrangements including inflatable tubs filled with ice to effectively treat heatstroke patients.
Dr Seema Balkrishna Wasnik, Head of Emergency Medicine at RML, spoke about the urgency and critical care measures in place and said that inflatable tubs filled with ice have been set up for heatstroke patients arriving at the hospital.
According to a ANI report, Dr Seema Balkrishna Wasnik, HOD, Emergency Medicine, RML, said, “At RML hospital, if any patient (of heat stroke) comes in critical situation he is taken to the red zone, intubated, we have inflatable tubs. We can still put the patient on a ventilator and also in the bathtub filled with ice and cold water and bring down the temperature simultaneously.”
Also Read:PM Modi sends medical team after journalist faints at rally
Wasnik stressed the importance of prompt treatment, stating, “The treatment of heatstroke should be started as soon as possible; otherwise, the mortality goes up to 80 per cent and if we manage the patient early, the mortality can go as down as 10 per cent.”
The India Meteorological Department (IMD) reported that temperatures exceeded 45 degrees Celsius at 41 locations across the country on May 30. In Delhi, Safdarjung recorded 45.6°C, Palam 46.4°C, Ridge 46.7°C, and Ayanagar reached 47.0°C, news agency ANI reported.
The India Meteorological Department said on Wednesday that the heatwave to severe heatwave conditions prevailing over Northwest and Central India are likely to reduce gradually from May 30.
It further said that hot and humid weather is very likely to prevail over isolated parts of Konkan and Goa on May 30 and 31.
The maximum temperatures were markedly above normal at a few places over Punjab and Haryana, Chandigarh, and Delhi on May 28, as per the IMD data.
Earlier, the IMD clarified that the maximum temperature of over 52 degrees Celsius recorded in Delhi on Wednesday was an “error in sensor or local factor.” The IMD in an official release stated that it is examining the data and sensors.
“The maximum temperature over Delhi NCR varied from 45.2 degrees Celsius to 49.1 degrees Celsius in different parts of city, Mungeshpur reported 52.9 degrees Celsius as an outlier compared to other stations. It could be due to an error in the sensor or the local factor. IMD is examining the data and sensors,” the release said.
Powered by WPeMatico

Puducherry- Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER) has invited proposals for competitive intramural research grants for faculty.
Intending to provide high-quality, innovative research ideas that are likely to be impactful, the competitive nature of research grants prepares our faculty to apply for extramural grants. Therefore, a call for this proposal will be issued every 3-6 months, an official circular stated.
The proposals can be in areas related to basic, translational, and clinical research. These will be reviewed by a Scientific Committee, including external experts if necessary. The projects will be prioritised based on-
1 Scientific merit: novelty, clarity of objectives and methodological rigour.
2 Feasibility, including timelines and resources required.
3 Applicability (direct or indirect) to health issues in the region, and likelihood of leading to continued future extramural-funded research on the particular topic in the Institute.
4 Projects with collaboration across disciplines.
It is to be noted that, the number of projects to be approved is flexible. Proposals with higher priority ranking will be funded. Funds will be disbursed on an annual basis through SFACTS, initially upon ethics clearance, and for 2″ years after evaluation of the annual progress report and receipt of the statement of expenditure.
Meanwhile, Principal investigators (PIs) applying for these grants must be permanent faculty members at (Puducherry or Karaikal). Co-investigators can be from within the Institute or from other institutions.
Furthermore, each proposal can have a budget of up to Rs. 6 lakhs and a duration of up to 2 years. A PI is eligible to get only one grant during a particular iteration, with a maximum of two concurrent intramural grants (approved at different times) at any time. Major equipment (costing, over Rs 50.000 each) or staff are not permitted.
APPLICATION PROCESS
Applications should be emailed to the official website of JIPMER as mentioned in the circular by June 20, 2024, and should also include-
1 A one-page cover letter with a self-explanatory proposal title, outlining the relevance.
2 A research proposal with title, justification/background, objectives, methodology, expected outcomes (in free format with the above headings; up to 5 pages; single-spaced; font size 12 with inter-paragraph space and 1″ margin all around), and budget on a separate page.
3 A short CV of the PI and external co-investigators (max 2 pages: only qualifications,
work experience, and details of up to 5 original research papers in Vancouver format).
4 For PI, a table listing all intramural/extramural grants over the last 5 years as PI (with project title, start/end dates, funding agency, and the amount sanctioned for the JIPMER centre) should be annexed.
However, proposals that were not funded in the previous cycle can also be submitted, with the suggested modifications, the circular lastly added.
To view the circular, click the link below
Powered by WPeMatico

Chennai: Granting relief to a Foreign Medical Graduate (FMG), who was denied to appear in the mandatory screening test for being underage at the time of MBBS admission abroad, the Madurai Bench of the Madras High Court recently directed the National Medical Commission (NMC) to allow the medico for appearing in the screening test.
Last year, Apex Medical Commission rejected his request to appear in the screening test. Following this, the student approached the Madras High Court seeking relief and filed a Public Interest Litigation (PIL) in this regard.
The concerned student joined the MBBS course in Ukraine on October 18, 2017. Back then, he was 11 days away from turning 18.
As per the existing rules, it is mandatory for the FMGs to clear the screening test i.e. the Foreign Medical Graduates Examination (FMGE) and undergo the compulsory internship in India. Only after fulfilling these requirements, the doctors become eligible to register and practice in India.
Therefore, after completing his medical education, the petitioner doctor applied to appear in the screening test conducted by NMC. However, the Commission rejected his request in October 2023 because he had not attained the minimum age of 18 when he joined the course, reports The New Indian Express
While considering the plea by the student, the Madras HC bench comprising Justice GR Swaminathan cited a ruling of a Division Bench of the Rajasthan High Court and observed that the refusal of an eligibility certificate for a medical course, because the candidate was not 18 years old, may not be justified.
“This case is different as Manikandan has completed his medical course. He is not seeking admission now, but wants to write the screening test,” observed the Court.
At this outset, the counsel for NMC pointed out that Manikandan had joined the overseas college without clearing the National Eligibility-cum-Entrance Test (NEET). However, the HC bench noted that the requirement of qualifying NEET for admission to medical colleges abroad became mandatory only from the 2019-2020 academic year.
The Court observed that if the concerned student had studied in India, he would have had to clear the NEET exam and would have met the age criteria.
The HC bench noted,
“But during the relevant time, he was not required to write NEET. He has completed his six-year course, and if he is denied relief at this point, it will ruin him.”
With this observation, the HC bench directed the NMC Secretary to issue the eligibility certificate, and hall ticket, and allow the petitioner student to participate in the screening test.
Powered by WPeMatico

Thiruvananthapuram: Doctors at KIMSHEALTH Trivandrum performed a rare procedure using a custom-made ‘Anaconda device’ – to treat a 79-year-old patient, who had a life-threatening aneurysm in the lower part of the aorta – the largest artery that carries blood out of the heart. If left unattended it would have ruptured any time leading to serious complications.
The patient, a native of Kollam, presented at the hospital with a hernia. On detailed evaluation, he was diagnosed with an 8 cm diameter abdominal aortic aneurysm — a bulging, weakened area in the wall of a blood vessel resulting in abnormal widening or ballooning.
“The aneurysm was angulated and situated very close to the artery of the kidney and bowel. Since there is no indigenous device available in the market capable of manoeuvring through the bends, the medical team decided to use a custom-made flexible stent graft,” said Dr Manish Kumar Yadav, Senior Consultant, Neuro Interventional Radiology, Imaging & Interventional Radiology.
Also Read:Adrenal tumour removed from 11-month-old child by keyhole surgery at KIMSHEALTH
The medical team performed a 10-hour-long procedure involving the deployment of the Anaconda device into the aneurysm, followed by cannulation and stenting of the abdominal branches. These branches are vital pathways to the liver, intestines, and kidneys, ensuring optimal blood flow and functionality.
“This marks the first time in the country that a flexible stent graft has been used to treat an abdominal aortic aneurysm. The device was customised according to the size and location of the aneurysm,” said Dr Manish. The patient left the hospital fully recovered. The successful outcome of this procedure not only addresses the immediate concern of the abdominal aortic aneurysm but also ensures the continued health and well-being of the patient by preserving vital organ functions, he added.
Dr Madhavan Unni, Senior Consultant & Chief Coordinator, Department of Imaging & Interventional Radiology, Dr Santhosh Joseph, Senior Consultant & Clinical Lead, Department of Neuro Interventional Radiology, Dr. Shaji Palangadan, Senior Consultant and Coordinator, Cardiothoracic Surgery, Dr. Subash S, and Dr. Anil Radhakrishnan Pillai, Consultants, Department of Cardio-Thoracic Anesthesia, were also part of this procedure.
Powered by WPeMatico

Punjab- Through a recent notice, Baba Farid University of Health Sciences (BFUHS) has informed about the revised dates of registration for B.Sc Nursing courses for the session 2024 and the schedule of the 1st round of online counselling.
REVISED REGISTRATION DATES
REVISED SCHEDULE OF 1st ROUND OF ONLINE COUNSELLING
|
S.NO |
EVENT |
REVISED DATE |
|
1 |
All PPMET (qualified ) candidates will submit online preferences of colleges/categories through the University website |
From 04-07-2024 to 08.07.2024 |
|
2 |
Display of provisional allotment/result. |
Upto 11.07.2024 |
|
3 |
Inviting objections to the provisional allotment (if any) by aspirants through Login ID. |
12.07.2024 |
|
4 |
In case there is any change in provisional allotment after considering objections the same will be displayed on the university website. |
13.07.2024 |
|
5 |
Candidates, to whom seats will be allocated, will report to the respective allotted college for joining, checking of documents/ eligibility, and Medical Checkup and will deposit fees through an online payment gateway available at the University website. |
13.07.2024 to 20.07.2024 |
Meanwhile, the revised schedule of 2nd round of online counselling will be intimated later on through the University’s official website.
To view the revised schedule, click the link below
Powered by WPeMatico
