Local application of Ivermectin ointment safe and effective therapy for patients with Demodex blepharitis: Study
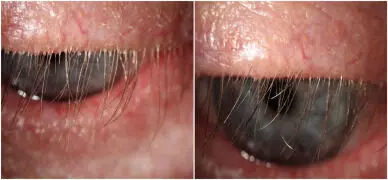
Local application of Ivermectin ointment safe and effective therapy for patients with Demodex blepharitis suggests a study published in Graefe’s Archive for Clinical and Experimental Ophthalmology.
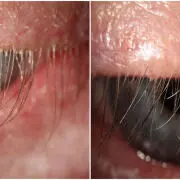
A study was done to evaluate the efficacy of topical ivermectin 1% ointment, for the treatment of Demodex blepharitis. A retrospective study was designed to review electronic medical records of patients seen between January 2017 and December 2022, who had a diagnosis of Demodex blepharitis, treated with topical ivermectin 1% with at least 6 months of follow-up (Centro de Ojos Quilmes, Buenos Aires, Argentina). The presence of collarettes was graded from 0 to 4. An imaging system (Keratograph) was used, to evaluate tear meniscus height (TMH), non-invasive tear break-up time (NIKBUT), and degree of conjunctival redness. In addition, the ocular surface disease index (OSDI) test was performed. Results were compared before and after ivermectin treatment, which was performed once a day for 2 months. Results: A total of 2157 patients (4314 eyes) were included. The mean age was 50.43 ± 15.3 years, and the follow-up time was 26.1 ± 8.5 months. No one discontinued treatment due to intolerance, although 14 cases (0.6 %) reported occasional discomfort. The grade of collarettes decreased with statistical significance, from 3.37 ± 0.7 to 0.1 ± 0.3 (p < 0.01), as well as conjunctival redness from 1.32 ± 0.3 to 0.94 ± 0.4 (p < 0.01) and OSDI score from 58.74 ± 17.9 to 17.1 ± 10.5 (p = 0.02). TMH and NIKBUT improved without statistical difference. Treatment with ivermectin 1% topical ointment, once daily for 2 months, was effective in reducing the presence of collarettes and in improving symptoms in patients with Demodex blepharitis.
Reference:
Valvecchia, F., Greco, L., Perrone, F. et al. Topical ivermectin ointment treatment of Demodex blepharitis: a 6-year retrospective study. Graefes Arch Clin Exp Ophthalmol 262, 1281–1288 (2024). https://doi.org/10.1007/s00417-023-06281-0
Keywords:
Local application, Ivermectin, ointment, safe, effective therapy, patients Demodex blepharitis, study, Graefe’s Archive for Clinical and Experimental Ophthalmology, Valvecchia, F., Greco, L., Perrone, Demodex, Ivermectin, Blepharitis, Ocular surface
Powered by WPeMatico