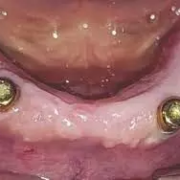
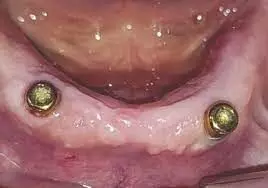
Laser bio-stimulation may enhance soft and hard peri implant tissues after 1-stage ridge splitting in narrow mandibular ridges: Study
Laser bio-stimulation may enhance soft and hard peri-implant tissues after 1-stage ridge splitting in narrow mandibular ridges suggests a study published in the Journal of Prosthetic Dentistry.
The management of patients with narrow-mandibular ridges who seek prosthetic rehabilitation is challenging. The purpose of this one-year preliminary clinical study was to compare the effects of laser biostimulation and placebo on peri-implant tissues for a 2-implant-retained mandibular polyetheretherketone (PEEK) overdenture on expanded narrow mandibular ridges. Eighteen completely edentulous participants were enrolled for mandibular ridge splitting in the canine regions, followed by expansion, the placement of implants, and the application of a bone graft. In the test group, laser therapy was applied labially and lingually at the surgical sites, while a placebo laser was used in the control group. PEEK overdentures retained by LOCATOR attachments were provided after 6 months. Clinical evaluations were performed using probing depth, plaque, bleeding, and gingival indices at insertion and 3, 6, and 12 months after insertion. Vertical bone loss (VBL) was evaluated with a periapical radiograph at insertion and 6 and 12 months later. The Mann-Whitney test was used to test the difference between the 2 different groups at each evaluation time (α=.05). The Friedman test was used, followed by the Wilcoxon signed rank test, to test the change over time in the same group, and the Bonferroni adjusted significance level was used for multiple comparisons. Results: Some clinical and radiographic parameters significantly increased with time in both groups (P<.001). Significant differences between the 2 groups were revealed in bleeding scores at 3 months (P=.006) and 6 months (P=.018). Also, significant differences between the 2 groups were observed in gingival scores at 3 months (P=.002), 6 months (P=.015), and 12 months (P=.019) after overdenture insertion in favor of the laser group. Peri-implant VBL was significantly higher in the non-laser group at 6 months (P=.015), and 12 months (P=.001). Within the limitations of this clinical study, respecting the small sample size and the short follow-up period, laser bio-stimulation after 1-stage ridge splitting in narrow mandibular ridges enhanced the soft and hard peri implant tissues when used with LOCATOR attachments and PEEK overdentures.
Reference:
El-Waseef FA, Helmy MA, Said Ahmed WM, Hegazy SA, El-Shaheed NH. Efficacy of laser biostimulation for mandibular narrow ridges treated with one-stage ridge splitting and two-implant overdentures: A one-year preliminary study. J Prosthet Dent. 2024 Apr 25:S0022-3913(24)00239-7. doi: 10.1016/j.prosdent.2024.03.045. Epub ahead of print. PMID: 38670908.
Keywords:
Laser, bio-stimulation, enhance, soft, hard peri implant, tissues, after, 1-stage, ridge splitting, narrow mandibular ridges, study , Journal of Prosthetic Dentistry, El-Waseef FA, Helmy MA, Said Ahmed WM, Hegazy SA, El-Shaheed NH
Powered by WPeMatico