FDA panel gives nod to blood test for colon cancer
Powered by WPeMatico
Powered by WPeMatico

Paris: Sanofi has announced that the U.S. Food and Drug Administration (FDA) has accepted for Priority Review the supplemental Biologics License Application (sBLA) for the investigational use of Sarclisa (isatuximab) in combination with bortezomib, lenalidomide and dexamethasone (VRd) for the treatment of patients with transplant-ineligible newly diagnosed multiple myeloma (NDMM).
If approved, Sarclisa would be the first anti-CD38 therapy in combination with standard-of-care VRd in newly diagnosed patients not eligible for transplant, which would be the third indication for Sarclisa in multiple myeloma. The target action date for the FDA decision is September 27, 2024. A regulatory submission is also under review in the European Union (EU).
Dietmar Berger, M.D., Ph.D., Chief Medical Officer, Global Head of Development at Sanofi said, “Despite recent advancements in multiple myeloma treatment, there remains a significant unmet need for new frontline therapies, particularly for transplant-ineligible patients who can face poor outcomes from the disease. The filing acceptances, as well as the FDA’s Priority Review designation, reinforce our confidence in Sarclisa as a potential best-in-class treatment and represent a critical step toward advancing this combination in a difficult-to-treat cancer.”
The sBLA, as well as the submission in the EU, is based on positive results from the IMROZ phase 3 clinical study evaluating the investigational use of Sarclisa in combination with standard-of-care VRd. In December 2023, the study met its primary endpoint at a planned interim analysis for efficacy, demonstrating statistically significant improvement in progression-free survival (PFS) with Sarclisa in combination with VRd compared with VRd alone in transplant-ineligible patients with NDMM. The safety and tolerability of Sarclisa observed in this study was consistent with the established safety profile of Sarclisa and VRd.
The IMROZ study is the fourth phase 3 study investigating Sarclisa combinations in NDMM patients to show superiority versus standard-of-care VRd and KRd, reinforcing its best-in-class potential. Results from the IMROZ study will also be featured during an oral presentation at the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting and during the plenary scientific session at the 2024 European Hematology Association (EHA) Annual Congress.
Priority Review is granted to regulatory applications seeking approval for therapies that have the potential to provide significant improvements in the treatment, diagnosis or prevention of serious conditions.
The investigational use of Sarclisa in combination with VRd in patients with transplant-ineligible NDMM is currently under clinical development, and its safety and efficacy for this indication have not been fully evaluated by any regulatory authority.
Powered by WPeMatico

Thiruvananthapuram: In response to numerous complaints of medical negligence against state-run hospitals, the Kerala government announced on Friday the implementation of medical audits across all its hospitals to address these issues. This initiative was revealed by State Health Minister Veena George.
Powered by WPeMatico
A recent retrospective study published in the recent issue of Nature Scientific Reports highlighted how the variability in risk factors such as uric acid and lipid levels can influence the development of complications in the patients with type 2 diabetes.
This research was conducted at a tertiary care hospital in Chengdu from 2013 to 2022 and analyzed electronic medical records of 369 diabetic patients to explore these associations. This comprehensive study focused on the variability of risk factors that was presented as the standard deviation (SD) and its impact on the diabetic complications. The study identified key factors that contribute to the complications by employing a binary logistic regression model.
One significant finding was that outpatient special disease management served as a protective factor against the development of complications with an odds ratio (OR) of 0.53 and a 95% confidence interval (CI) of 0.29 to 0.10. This management approach was particularly effective in preventing diabetic peripheral neuropathy with an OR of 0.51 and a 95% CI of 0.30 to 0.86.
Also, the variability in total cholesterol levels (TC-SD) was observed to be a significant risk factor. The patients with higher variability in their cholesterol levels were more likely to develop complications with an OR of 2.42 and a 95% CI of 1.18 to 4.97. This variability also posed a specific risk for diabetic peripheral vasculopathy with an OR of 2.50 and a 95% CI of 1.25 to 5.02.
The findings of this study underline the dual importance of glycemic control and lipid regulation in managing type 2 diabetes. While outpatient special disease management proves beneficial in reducing the risk of complications as the management of lipid levels is crucial for patients, especially the patients who were not under such specialized care. Outpatient special disease management significantly reduce the risk of overall complications and specifically diabetic peripheral neuropathy. High variability in total cholesterol levels is linked to an elevated risk of both general diabetic complications and diabetic peripheral vasculopathy.
The study advocates for a comprehensive approach to diabetes care by emphasizing the regulation of lipid levels alongside glycemic control to delay or prevent the onset of complications. The findings could influence the future guidelines and patient management strategies in diabetes care which could potentially lead to better outcomes for patients.
Source:
Chen, M., Pu, L., Gan, Y., Wang, X., Kong, L., Guo, M., Yang, H., Li, Z., & Xiong, Z. (2024). The association between variability of risk factors and complications in type 2 diabetes mellitus: a retrospective study. In Scientific Reports (Vol. 14, Issue 1). Springer Science and Business Media LLC. https://doi.org/10.1038/s41598-024-56777-w
Powered by WPeMatico
Researchers have found that high-dose influenza vaccine (HD-IV) significantly reduces the rates of pneumonia and influenza (P&I) and all-cause hospitalizations in adults aged 65 and older compared to the standard-dose influenza vaccine (SD-IV). This study provides crucial insights into the relative effectiveness of HD-IV, particularly for severe clinical outcomes. This study was published in the Journal Of Infection by Kristoffer G. and colleagues.
Influenza poses a significant health risk, especially for older adults, leading to severe complications such as pneumonia and increased hospitalizations. This study aimed to summarize the current evidence on the effectiveness of HD-IV versus SD-IV in mitigating these severe outcomes in the elderly population. The study’s objective was to assess whether HD-IV offers superior protection against P&I hospitalizations, all-cause hospitalizations, and all-cause death compared to SD-IV.
A prespecified meta-analysis was conducted, encompassing five randomized controlled trials with a total of 105,685 participants aged 65 years and older. The analysis evaluated the relative vaccine effectiveness (rVE) of HD-IV versus SD-IV using fixed-effects models with the inverse variance method. The primary outcomes measured were the rates of P&I hospitalization, all-cause hospitalization, and all-cause death.
HD-IV was associated with a 23.5% reduction in P&I hospitalizations compared to SD-IV (rVE: 23.5%, [95% CI: 12.3% to 33.2%]).
The rate of all-cause hospitalizations was reduced by 7.3% in the HD-IV group compared to the SD-IV group (rVE: 7.3%, [95% CI: 4.5% to 10.0%]).
No significant difference was observed in all-cause death rates between HD-IV and SD-IV (rVE: 1.6%, [95% CI: -2.0% to 5.0%]).
The findings suggest that HD-IV is more effective than SD-IV in reducing severe clinical outcomes like P&I and all-cause hospitalizations in adults aged 65 and older. The consistent protection across different subgroups underscores the importance of considering HD-IV for elderly populations, especially during flu seasons with high rates of complications. Despite the lack of significant difference in all-cause death rates, the reduction in hospitalization rates is a compelling argument for the use of HD-IV.
Sensitivity analyses, which omitted trials with participants sharing the same comorbidity, trials with 100 or more events, and using random-effects models, yielded comparable estimates for all outcomes, reinforcing the robustness of the findings.
This study concludes that HD-IV significantly reduces the incidence of P&I and all-cause hospitalizations compared to SD-IV in adults aged 65 and older. While no significant difference was observed in all-cause death rates, the evidence supports the use of HD-IV to mitigate severe clinical outcomes in the elderly. These findings, drawn from several randomized trials, highlight the potential benefits of HD-IV and suggest the need for further research in fully powered, individually randomized trials.
Reference:
Skaarup, K. G., Lassen, M. C. H., Modin, D., Johansen, N. D., Loiacono, M. M., Harris, R. C., Lee, J. K. H., Dufournet, M., Vardeny, O., Peikert, A., Claggett, B., Solomon, S. D., Jensen, J. U. S., & Biering-Sørensen, T. (2024). The relative vaccine effectiveness of high-dose vs standard-dose influenza vaccines in preventing hospitalization and mortality: A meta-analysis of evidence from randomized trials. The Journal of Infection, 106187, 106187. https://doi.org/10.1016/j.jinf.2024.106187
Powered by WPeMatico

Spain: In a groundbreaking development for cardiovascular health, the results of the PREDIMAR trial have illuminated the potential of a Mediterranean diet supplemented with extra-virgin olive oil in reducing the risk of tachyarrhythmia recurrence following atrial fibrillation (AF) ablation. Conducted by a team of researchers, this late-breaking clinical trial represents a significant milestone in the quest for effective interventions to improve outcomes for AF patients.
The study found that among atrial fibrillation patients who had ablation, those assigned to a Mediterranean diet enriched with extra-virgin olive oil were less likely to have arrhythmia recurrence versus those with no dietary restrictions.
According to findings from the trial presented at Heart Rhythm 2024, the difference was most pronounced for patients with paroxysmal AF at baseline.
Atrial fibrillation, the most common form of arrhythmia, affects millions worldwide and poses significant challenges due to its association with increased risk of stroke, heart failure, and other cardiovascular complications. Catheter ablation has emerged as a cornerstone in AF management, but recurrence of tachyarrhythmia post-ablation remains a concern, necessitating novel approaches to optimize long-term outcomes.
Strategies for improving results for AF ablation have focused on pharmacology and technology but have not extensively looked at lifestyle and diet. The PREDIMAR study sought to determine whether a Mediterranean diet enriched with extra-virgin olive oil could be integrated into treatment plans for paroxysmal AF patients undergoing catheter ablation to reduce their risk of tachyarrhythmias recurrence.
The trial, led by researchers at the University of Navarra, University Hospital HM Monteprincipe, Virgen de las Nieves Hospital, and General Hospital of Alicante in Spain, included 720 AF patients treated with ablation. They were randomized in a 1:1 ratio; one group received advice to follow a Mediterranean diet enriched with extra-virgin olive oil or their freely selected diet.
The trial followed all patients for 18 months, with medical visits every 3 to 6 months. They also received an electrocardiogram recording device to use once a week and if they had any symptoms.
The researchers reported the following findings:
· After 18 months, the results showed a 10% relative reduction in the risk of tachyarrhythmia recurrence in patients following the Mediterranean diet enriched with extra-virgin olive oil compared to patients who freely selected their diet. This reduction was even higher in patients with paroxysmal AF at baseline (before ablation).
· These patients experienced a 31% relative reduction in the risk of recurrence with the Mediterranean diet intervention compared to the freely selected diet.
“Patients frequently ask me about what diet and lifestyle changes are most impactful after being diagnosed with atrial fibrillation or following an ablation,” said Maria Teresa Barrio Lopez, M.D., PhD, Cardiologist, University Hospital HM Monteprincipe in Spain.
“Seeing the results of the PREDIMAR trial, I feel confident in recommending the Mediterranean diet enriched with extra-virgin olive oil to patients to help reduce the risk of recurrences.”
The implementation of a dietetic program for patients holds the potential to not only lower arrhythmia recurrence but also impact hospital and emergency admissions, the risk of stroke associated with AF, and additional ablation procedures.
Reference:
“Late Breaking Clinical Trials: Mediterranean diet enriched with extra-virgin olive oil reduced risk of tachyarrhythmia recurrence after atrial fibrillation ablation: the PREDIMAR trial [Saturday, May 18, 2024, at 2:00 pm ET]
Powered by WPeMatico
Late last year, geneticist Marlena Fejzo and colleagues made the discovery that morning sickness’s most serious presentation, hyperemesis gravidarum (HG), is caused by the hormone GDF15, not human chorionic gonadotropin as previously thought.
In a peer-reviewed opinion article publishing May 22 in the journal Trends in Molecular Medicine, Fejzo dispels common morning sickness myths and discusses potential treatments, including sensitizing people to GDF15 prior to pregnancy, similar to the way we treat allergies.
“HG can be life threatening and is associated with adverse outcomes that need to be taken seriously,” says Fejzo (@DrFejzo) of the Keck School of Medicine of the University of Southern California. “Now that we know that GDF15 is the most likely cause of HG, we are on the cusp of having treatments that target this hormonal pathway and end the suffering.”
Myth 1: Severe morning sickness is harmless and normal
Pregnant people with HG are essentially starving, Fejzo says, and an increasing number of studies have demonstrated that this has serious short- and long-term clinical implications for both the parent and child. HG is a top predictor of postnatal depression, and 26% of pregnant people with HG report suicidal ideation while 18% meet the full criteria for post-traumatic stress disorder.
For the child, HG is associated with preterm birth, low birth weight, and later in life, autism spectrum disorder, ADHD, depression, social problems, in addition to an increased risk of childhood cancer and respiratory and cardiovascular disease. Still, pregnant people with the condition are often dismissed by their clinicians and families.
“It really is like a teratogen in pregnancy, a factor which interferes with normal fetal development, but it’s still not taken seriously by a lot of medical professionals,” Fejzo says. “A lot of people are brushed off and told, ‘oh that’s normal, it’s okay, just don’t take your pre-natal vitamins; you don’t need them.’”
At its most extreme, individuals with HG can develop Wernicke encephalopathy, a life-threatening swelling of the brain due to thymine (vitamin B1) deficiency. Since individuals with HG can have trouble even swallowing vitamins, the American College of Obstetricians and Gynecologists currently recommends that they replace broad spectrum prenatal vitamins with folic acid, but Fejzo warns that this is likely insufficient, and that thiamine supplementation is also warranted for individuals with HG.
“I believe all women who have hyperemesis should be given vitamin B1 to avoid this serious brain swelling that can lead to permanent brain damage and often leads to fetal death,” Fejzo says.
Myth 2: Morning sickness is caused by human chorionic gonadotropic hormone (hCG) or is psychosomatic
Though it was long thought that morning sickness is caused by hCG, the recent breakthrough has shown that HG’s main cause is actually the hormone GDF15, which is part of a normal stress response. Usually, GDF15 is expressed only in very small amounts, but during early pregnancy it spikes by a huge amount, then wanes, and finally rises again during the third trimester.
A recent Nature study co-authored by Fejzo showed that individuals who suffer from HG can have genetic variants that causes them to have lower levels of circulating GDF15 prior to pregnancy, which makes them extra sensitive when they become pregnant and are suddenly exposed to high levels. This finding has clinical implications for preventing and treating HG, since preliminary research suggests that it might be safe to manipulate GDF15 during or even prior to pregnancy.
“GDF15 may be safe to manipulate in pregnancy or even prior to pregnancy,” says Fejzo. “If we can increase levels of GDF15 before someone becomes pregnant, that might desensitize them, similar to how we try to desensitize people to allergens who have severe allergies,” says Fejzo. “And during pregnancy, we may be able to minimize or get rid of symptoms by blocking GDF15 or its receptors in the brain stem.”
Myth 3: Only humans experience morning sickness
Nausea and appetite loss during gestation is not a uniquely human trait—these symptoms have been observed throughout the animal kingdom, from monkeys, dogs, and cats, to chickens, vipers, and octopuses.
“I always think it’s interesting that the recommendation for cats is that if they’re unable to eat for a day, you should contact your veterinarian, but we don’t have that recommendation out there for women with hyperemesis,” says Fejzo. “If you call your doctor’s office and say you haven’t eaten for a day, they’ll say, ‘that’s normal’ and won’t do anything. There’s more proactive care for cats than humans.”
In addition to preventing ingestion of harmful foods, Fejzo speculates that pregnancy-induced nausea likely evolved to prevent dangerous foraging trips.
“This condition likely evolved because it was probably beneficial to avoid going out searching for food during pregnancy,” says Fejzo. “That may still be true for animals, but people don’t need this anymore, so let’s end the suffering once and for all if we can.”
Now, Fejzo is working toward developing and testing the proposed GDF15-based treatments. She also plans to investigate other genes and variants of GDF15 that might contribute to HG.
References: Marlena Schoenberg Fejzo Published:May 22, 2024 DOI: https://doi.org/10.1016/j.molmed.2024.04.006
Powered by WPeMatico
Researchers have found that pamrevlumab, a monoclonal antibody, does not significantly slow the decline in lung function for patients with idiopathic pulmonary fibrosis (IPF) compared to a placebo. This conclusion comes from a phase 3 randomized clinical trial aimed at assessing the efficacy and safety of pamrevlumab for treating IPF. The study was published in JAMA by Ganesh Raghu and colleagues.
Current treatments for idiopathic pulmonary fibrosis, such as nintedanib and pirfenidone, slow the progression of the disease but are often associated with adverse events that can affect medication adherence. Pamrevlumab, a fully human monoclonal antibody that inhibits connective tissue growth factor, showed promise in phase 2 trials by attenuating disease progression with minimal adverse effects. This phase 3 study aimed to evaluate its effectiveness and safety in a larger patient population.
The study included 356 patients aged 40 to 85 years with IPF who were not receiving nintedanib or pirfenidone at enrollment. Patients were recruited from 117 sites across 9 countries between July 18, 2019, and July 29, 2022. The last follow-up encounter occurred on August 28, 2023. Participants were randomized to receive either pamrevlumab (30 mg/kg intravenously every 3 weeks; n = 181) or a placebo (n = 175) for 48 weeks.
The primary outcome was the absolute change in forced vital capacity (FVC) from baseline to week 48. Secondary outcomes included time to disease progression (defined as a decline of ≥10% in predicted FVC or death), and exploratory outcomes encompassed patient-reported symptoms. Adverse events were also recorded.
• Among the 356 patients (mean age, 70.5 years; 72.5% male; 62.1% White), 277 (77.8%) completed the trial. T
• The study found no significant difference in the primary outcome between the pamrevlumab and placebo groups.
• The least-squares mean change in FVC from baseline to week 48 was -260 mL (95% CI, -350 to -170 mL) in the pamrevlumab group versus -330 mL (95% CI, -430 to -230 mL) in the placebo group, resulting in a mean between-group difference of 70 mL (95% CI, -60 to 190 mL; P = .29).
• No significant difference in FVC change from baseline to week 48 between pamrevlumab and placebo groups (70 mL difference; 95% CI, -60 to 190 mL; P = .29).
• No significant differences between groups in any secondary outcomes.
• Treatment-related adverse events occurred in 88.4% of patients in the pamrevlumab group and 86.3% in the placebo group.
• Serious adverse events were reported in 28.2% of the pamrevlumab group and 34.3% of the placebo group.
• Mortality rates were similar, with 23 deaths in each group (12.7% vs. 13.1%).
Among patients with idiopathic pulmonary fibrosis, pamrevlumab did not result in a statistically significant difference in the primary outcome of FVC decline from baseline to week 48 compared to placebo. These results suggest that pamrevlumab may not offer additional benefits over existing treatments, underscoring the need for continued research into more effective therapies for IPF.
Reference:
Raghu, G., Richeldi, L., Fernández Pérez, E. R., De Salvo, M. C., Silva, R. S., Song, J. W., Ogura, T., Xu, Z. J., Belloli, E. A., Zhang, X., Seid, L. L., Poole, L., Bowler, S., Corte, T., Holmes, M., Thien, F., Wheatley, J., Choi, S.-M., Chung, M.-P., … ZEPHYRUS-1 Study Investigators. (2024). Pamrevlumab for idiopathic pulmonary fibrosis: The ZEPHYRUS-1 randomized clinical trial. JAMA: The Journal of the American Medical Association. https://doi.org/10.1001/jama.2024.8693
Powered by WPeMatico

Gestational weight gain may mediate the effect of exercise on the prevention of incident macrosomia, suggests a study published in the BMC Pregnancy and Childbirth.
They sought to investigate the impact of individualized exercise guidance during pregnancy on the incidence of macrosomia and the mediating effect of gestational weight gain (GWG). A randomized clinical trial was conducted from December 2021 to September 2022 to compare the effects of standard prenatal care with individualized exercise guidance on the incidence of macrosomia. Results: In all, 312 singleton women were randomized into an intervention group (N = 162) or a control group (N = 150). Participants who received individualized exercise guidance had a significantly lower incidence of macrosomia (3.73% vs. 13.61%, P = 0.002) and infants large for gestational age (9.94% vs. 19.73%, P = 0.015). However, no differences were observed in the rate of preterm birth (1.86% vs. 3.40%, P = 0.397) or the average gestational age at birth (39.14 ± 1.51 vs. 38.69 ± 1.85, P = 0.258). Mediation analysis revealed that GWG mediated the effect of exercise on reducing the incidence of macrosomia. Individualized exercise guidance may be a preventive tool for macrosomia, and GWG mediates the effect of exercise on reducing the incidence of macrosomia. However, evidence does not show that exercise increases the rate of preterm birth or affects the average gestational age at birth.
Reference:
Yang, X., Wang, G., Liu, N. et al. Mediating effect of gestational weight gain on the preventive effect of exercise during pregnancy on macrosomia: a randomized clinical trial. BMC Pregnancy Childbirth 24, 384 (2024). https://doi.org/10.1186/s12884-024-06527-7
Powered by WPeMatico
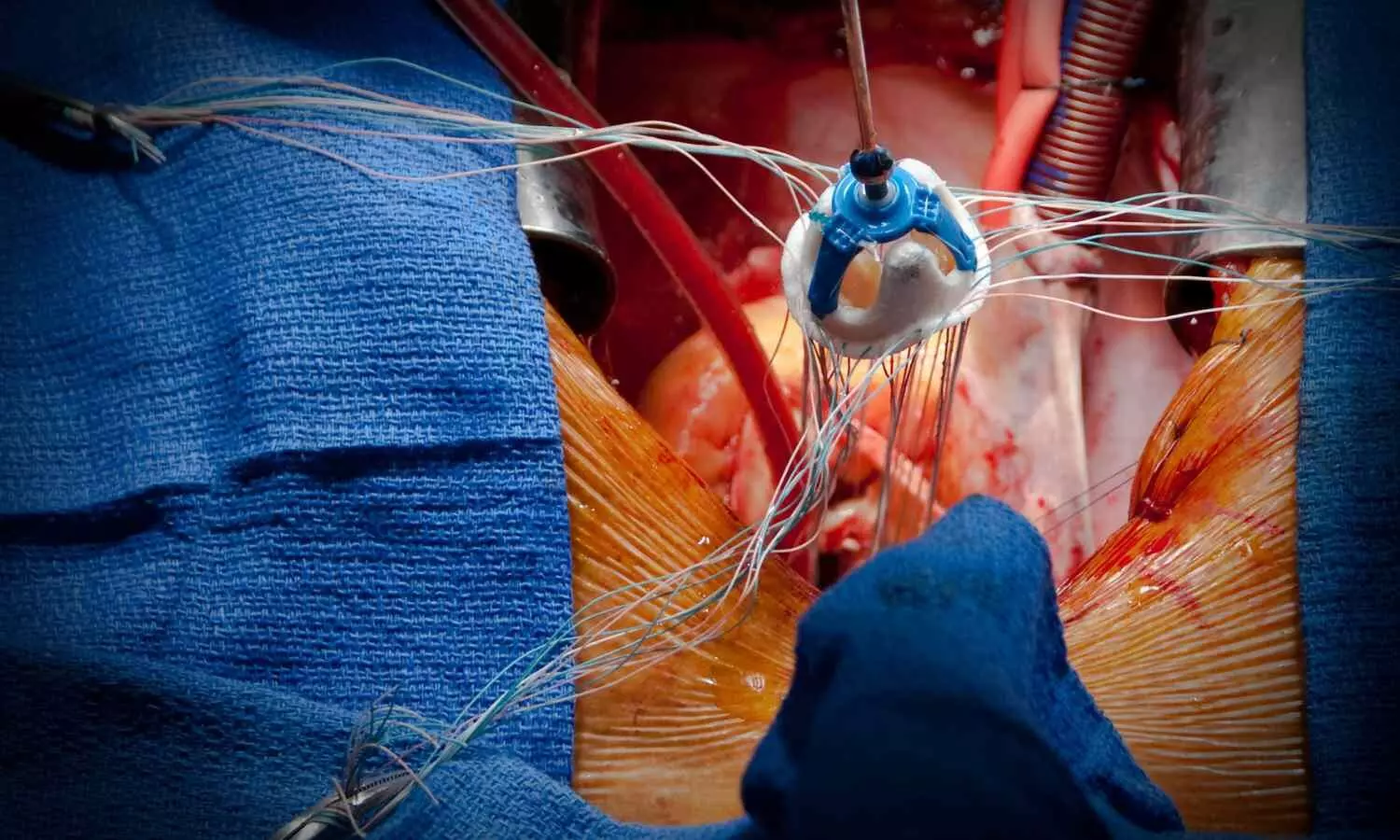
The Course Directors have selected 3 major Late Breaking Trials (LBTs) that will be presented for the first time during the 2024 edition of EuroPCR. These trials were selected on account of their design, outcomes and potential to influence daily clinical practice. Among them is the LANDMARK trial.
Key randomized controlled trials have compared surgical aortic valve replacement (SAVR) with transcatheter aortic valve implantation (TAVI) using one of two commercially available transcatheter heart valves (THVs) – either an intra-annular balloon-expandable valve (BEV) (Edwards Sapien, Sapien XT or Sapien 3) or a supra-annular self-expanding valve (SEV) (Medtronic CoreVavle, Evolut-R or Evolut Pro). Other THV have been introduced subsequently and head-to-head comparisons with established BEV or SEV platforms have frequently demonstrated less favorable efficacy and safety profiles.
Myval is a novel BEV whose key differentiating characteristic is the availability of a wider range of prosthesis sizes (with 1.5 mm increments versus 3 mm for comparators), allowing precise matching of the implanted valve with a patient’s specific anatomy. The LANDMARK randomized controlled trial investigated its early safety and efficacy in comparison with two widely adopted contemporary THVs (the Edwards SAPIEN BEV and Medtronic Evolut SEV).
The primary hypothesis was non-inferiority of the Myval THV and the primary safety and efficacy endpoint at 30-day follow-up was the composite of all-cause death, any stroke, life-threatening or disabling bleeding, stage 2 or 3 acute kidney injury, major vascular complications, moderate or severe paravalvular leak and new permanent pacemaker implantation (all defined according to VARC-3 criteria). Patients were included if considered clinically and anatomically suitable for a transfemoral TAVI procedure by their respective Heart Teams. Bicuspid aortic valve phenotypes were included and there were no restrictions related to estimated surgical risk.
A total of 768 patients (mean age 80 years) at low surgical risk undergoing transfemoral TAVI were randomised 1:1 to implantation of a Myval THV or an alternative contemporary TAVI device (BEV or SEV).
At 30 days, the primary composite endpoint occurred in 24.7% and 27.6 % of the Myval and control groups, respectively, denoting non-inferiority of the Myval THV compared to other contemporary THVs (risk difference -2.7%; one-sided upper 95% CI 3.6%; p<0·0001 for non-inferiority). There were no significant differences in any individual components of the primary endpoint, and no differences in technical and device success (defined according to VARC-3 criteria). Secondary endpoints, including rates of pacemaker implantation and improvements in hemodynamic parameters (mean pressure gradient and effective orifice area) were also similar in both study arms.
Previous head-to-head comparisons of new THVs with the established Edwards BEVs or Corevalve/Evolut SEVs confirmed that there is no class effect regarding the clinical benefits that patients can expect after TAVI, but demonstrated inferior safety and efficacy. Validation of the non-inferiority of the MyVal platform in comparison with the two most frequently used contemporary THVs in the LANDMARK TRIAL is therefore a significant finding. Importantly, the characteristics of the patients included in this trial reflect contemporary practice, incorporating patients at low and intermediate surgical risk, and 6.0-7.5% with a bicuspid aortic valve.
Although these short-term outcomes are promising, longer-term information concerning hemodynamic performance and risk of structural valve deterioration will be required to confirm durability of the Myval THV in comparison with contemporary valves. Follow up out to 10 years is planned to address this key question.
Powered by WPeMatico
