Phytochemicals May Aid Neurological Disorder Treatment by Impacting Gut-Brain Axis: Study

Powered by WPeMatico

Powered by WPeMatico
Researchers have found that prophylactic azithromycin does not improve survival rates or reduce the incidence of chronic lung disease of prematurity (CLD) in preterm infants born at less than 30 weeks’ gestation, according to the results of the AZTEC trial. This multicenter, double-blind, randomized, placebo-controlled trial challenges previous findings and suggests that azithromycin should not be recommended for preventing CLD in this population. This study was published in The Lancet Respiratory Medicine journal by John Lowe and colleagues.
Chronic lung disease of prematurity (CLD), a major health concern in preterm infants, can lead to significant morbidity and mortality. There has been conflicting evidence on whether macrolide antibiotics like azithromycin could potentially reduce the rates of CLD in at-risk infants, particularly those colonized with pulmonary Ureaplasma spp..
The AZTEC trial was conducted across 28 neonatal intensive care units in the UK. The study included infants born at less than 30 weeks’ gestation who had received non-invasive or invasive respiratory support within 72 hours of birth. Infants were randomly allocated to either receive intravenous azithromycin or a placebo, with masking to ensure blinding. The primary outcome assessed was survival without moderate or severe CLD at 36 weeks’ postmenstrual age.
The key findings of the study were as follows:
Survival without moderate or severe CLD occurred in 42% of infants in the azithromycin group and 45% in the placebo group (aOR 0.84, 95% CI 0.55–1.29, p=0.43).
Seven serious adverse events were reported in the azithromycin group (5 severe, 2 moderate), while 6 occurred in the placebo group (2 severe, 2 moderate, 2 mild).
The researchers concluded that the use of azithromycin did not significantly improve survival without CLD, regardless of the presence of pulmonary Ureaplasma spp. colonization.
Given the lack of efficacy in improving outcomes, azithromycin should not be recommended for preventing CLD in preterm infants at risk. These findings have important implications for clinical practice, highlighting the need for alternative approaches in managing and preventing CLD in this vulnerable population.
The AZTEC trial provides clear evidence that prophylactic azithromycin is not beneficial for preterm infants at risk of chronic lung disease of prematurity. Clinicians should consider other strategies for managing this condition in infants born at less than 30 weeks’ gestation.
Reference:
Lowe, J., Gillespie, D., Aboklaish, A., Lau, T. M. M., Consoli, C., Babu, M., Goddard, M., Hood, K., Klein, N., Thomas-Jones, E., Turner, M., Hubbard, M., Marchesi, J., Berrington, J., & Kotecha, S. (2024). Azithromycin therapy for prevention of chronic lung disease of prematurity (AZTEC): a multicentre, double-blind, randomised, placebo-controlled trial. The Lancet. Respiratory Medicine. https://doi.org/10.1016/s2213-2600(24)00079-1
Powered by WPeMatico

Powered by WPeMatico

Intravenous glibenclamide, a drug capable of reducing cerebral edema, did not improve functional outcome at three months in patients with large hemispheric infarction, a new study shows. The international randomised controlled trial did however confirm that the drug is safe and that there may be a beneficial signal in less severely affected stroke patients.
The CHARM study, presented today at the European Stroke Organisation Conference (ESOC) 2024, was a global, randomised, double-blind, placebo-controlled trial conducted at 141 hospitals. Patients who presented with large hemispheric infarction within 10 hours of last seen well were randomised to intravenous glibenclamide or placebo. The researchers primarily focused on the functional outcomes in both groups at three months, but also studied safety outcomes.
The group of patients who received intravenous glibenclamide (n=217 patients) did not have a lower likelihood of a poor functional outcome (common odds ratio [OR] 1.17; 95% confidence interval [CI] 0.80, 1.71) at three months, when compared to placebo (n=214 patients). The rates of serious adverse events were high in both groups, consistent with critical illness of the large stroke population.
Dr. W. Taylor Kimberly, one of the lead investigators on the study, from Massachusetts General Hospital, Boston, believes there is reason for optimism following CHARM. “We have learned a lot about glibenclamide during our 14-year journey from bench to bedside. When it comes to large hemispheric infarctions, we have wondered about a ceiling effect. How big a stroke is too big? Although we must be cautious in interpreting subgroup analyses, we have seen some promising signals in patients with medium-large stroke volumes (80-130 mL).”
Cerebral edema is a significant complication after ischemic stroke that can lead to neurological deterioration and death, especially in patients with large hemispheric infarction. The damage caused by stroke disrupts the blood-brain barrier and alters the function of ion channels, resulting in excess water accumulation and lesional swelling. Preclinical data and prior clinical trials have shown that glibenclamide can reduce cerebral edema following stroke by regulating the opening of ion channels through certain receptor proteins, the so- called surfonylurea receptor 1 (SUR1) proteins.
Dr Kevin Sheth, also one of the lead authors, from Yale School of Medicine, New Haven, adds, “We know the potential of glibenclamide through our translational research. However, getting everything right in a clinical stroke landscape that is evolving is challenging. It is very exciting to see consistent signals of potential drug effect across these various trial cohorts so that we can inform the next trial.”
Reference:
INTRAVENOUS GLIBENCLAMIDE FOR LARGE HEMISPHERIC INFARCTION: RESULTS FROM THE CHARM PHASE 3 TRIAL. Presented at the European Stroke Organisation Conference; 15 May 2024; Basel, Switzerland.
Powered by WPeMatico

Nearly three-quarters of the United States population receives drinking water that contains fluoride, a practice that began in 1945 to help prevent tooth decay. But recent studies suggest that fluoride exposure can cause harm to a fetus if consumed during pregnancy, a critical period for brain development.
A new study, led by researchers at the Keck School of Medicine of USC and funded in part by the National Institutes of Health, analyzed more than 220 mother-child pairs, collecting data on fluoride levels during pregnancy and child behavior at age three. The researchers found that a 0.68 milligram per liter increase in fluoride exposure was associated with nearly double the chance of a child showing neurobehavioral problems in a range considered close to or at a level to meet the criteria for clinical diagnosis.
The findings were just published in JAMA Network Open.
“Women with higher fluoride exposure levels in their bodies during pregnancy tended to rate their 3-year-old children higher on overall neurobehavioral problems and internalizing symptoms, including emotional reactivity, anxiety and somatic complaints,” said Tracy Bastain, PhD, an associate professor of clinical population and public health sciences and senior author of the study.
These population-level findings add to existing evidence from animal studies showing that fluoride can harm neurodevelopment, as well as data from studies conducted in Canada, Mexico and other countries showing that prenatal exposure to fluoride is linked with a lower IQ in early childhood. The researchers hope the new findings help convey the risks of fluoride consumption during pregnancy to policymakers, health care providers and the public.
“This is the first U.S.-based study to examine this association. Our findings are noteworthy, given that the women in this study were exposed to pretty low levels of fluoride-levels that are typical of those living in fluoridated regions within North America,” said Ashley Malin, PhD, an assistant professor of epidemiology at the University of Florida’s College of Public Health and Health Professions and College of Medicine and lead author of the present study. Malin conducted the research in part as a postdoctoral scholar at the Keck School of Medicine.
Data for the study came from the Maternal and Developmental Risks from Environmental and Social stressors (MADRES) Center for Environmental Health Disparities at the Keck School of Medicine. MADRES follows predominantly Hispanic families in Los Angeles from pregnancy throughout childhood.
“The overall goal of MADRES is reducing the effects of environmental contaminants on the health and well-being of marginalized communities,” said Bastain, who co-directs MADRES.
The researchers analyzed 229 mother-child pairs, calculating fluoride exposure from urine samples collected during the third trimester of pregnancy. Most urine samples were collected from fasting women, which improves the accuracy of chemical testing. Children were then assessed at age three using the Preschool Child Behavior Checklist, which uses parent reports to measure a child’s social and emotional functioning.
Children exposed to an additional 0.68 milligrams per liter of fluoride in the womb were 1.83 times more likely to show behavioral problems considered to be clinically significant or borderline clinically significant. Specifically, children exposed to more fluoride had more problems with emotional reactivity, somatic complaints (such as headaches and stomachaches), anxiety and symptoms linked to autism.
No association was found with several other neurobehavioral symptoms, including “externalizing behaviors” such as aggression and attention problems.
Currently, no official recommendations exist for limiting fluoride consumption during pregnancy, but the researchers hope these findings can help stimulate change.
]“There are no known benefits to the fetus from ingesting fluoride,” Malin said. “And yet now we have several studies conducted in North America suggesting that there may be a pretty significant risk to the developing brain during that time.”
Next, the research team will explore how exposure to fluoride during pregnancy may impact brain development among infants in the MADRES study. Additional studies in other regions of the country can also help determine the extent of the problem and the best way forward, Bastain said.
“While this is the first U.S.-based study of fluoride exposure during pregnancy, more studies are urgently needed to understand and mitigate the impacts in the entire U.S. population,” she said.
Reference:
Malin AJ, Eckel SP, Hu H, et al. Maternal Urinary Fluoride and Child Neurobehavior at Age 36 Months. JAMA Netw Open. 2024;7(5):e2411987. doi:10.1001/jamanetworkopen.2024.11987.
Powered by WPeMatico
Menopause linked to mandibular condylar trabecular bone loss affecting periodontal, orthodontic, and implant placement in elderly females suggests a study published in the Journal of Oral and Maxillofacial Surgery.
There are conflicting reports on the effects of decreased estrogen levels on mandibular bone microarchitecture. Whether these effects are consistent throughout the mandible is unclear and may have important implications for treatment planning. The goal of this study was to evaluate trabecular and cortical bone microstructure in the mandibular condyle and the mandibular basal bone and compare these sites between premenopausal and postmenopausal women. Participants were recruited for a cross-sectional cohort study at Columbia University Irving Medical Center. Each participant had cone-beam computed tomography taken of their mandibular condyles and the basal bone. Exclusion criteria for the population included a) current chemotherapy or immunotherapy; b) history of bisphosphonate or other osteoporosis therapy; and c) currently pregnant, nursing, or on hormonal birth control. Results: The premenopausal and postmenopausal groups each had 31 participants, with the following average age: premenopausal = 43.9 ± 6.9 versus postmenopausal = 57.5 ± 7.6 years old; P < .001, and estrogen levels: premenopausal = 91.77 ± 80.13 pg/ml versus postmenopausal = 41.44 ± 61.62 pg/ml; P < .01). Postmenopausal women had significantly greater condylar trabecular separation (0.61 ± 0.18 vs 0.47 ± 0.11 mm; P < .001) and lower trabecular number (1.03 ± 0.18 vs 1.21 ± 0.19 mm−1; P < .001) compared to premenopausal women. There were no significant differences in the basal bone microarchitectural parameters between the menopausal groups. Menopause is associated with mandibular condylar trabecular bone loss but has minimal effects on the mandibular basal bone. This may have important ramifications for treatment planning in advanced-age individuals.
Reference:
Levit M, Finn T, Sachadava S, Matsumura S, Shah J, Cantos A, Yin MT, Wadhwa S. Menopause-Associated Changes in Mandibular Bone Microarchitecture Are Site-Specific. J Oral Maxillofac Surg. 2024 Apr;82(4):485-493. doi: 10.1016/j.joms.2024.01.015. Epub 2024 Feb 1. PMID: 38341183; PMCID: PMC11010363.
Keywords:
Menopause, mandibular, condylar, trabecular bone loss, affecting, periodontal, orthodontic, implant placement, elderly females, study, Journal of Oral and Maxillofacial Surgery, Levit M, Finn T, Sachadava S, Matsumura S, Shah J, Cantos A, Yin MT, Wadhwa S
Powered by WPeMatico
Effects of Major Liver Resections on Coagulation – Recently published paper discusses the effects of major liver resections on the synthesis of coagulants and anticoagulants, resulting in a shift in the balance of coagulation and thrombosis. Bleeding is a significant concern postoperatively, and delayed initiation of pharmacological prophylaxis for venous thromboembolism (VTE) increases the risk. The removal of epidural catheters involves the requirement of a near-normal INR before removal, which has potential risks. Thromboelastography (TEG) is a sensitive test for evaluating coagulation, and the study aims to assess its use in liver resection compared to conventional coagulation tests (prothrombin time [PT], INR, and activated partial thromboplastin time [aPTT]). Value of Thromboelastography (TEG) in Liver Surgery A single-arm trial was conducted on patients undergoing major liver resections to evaluate the serial changes in conventional coagulation tests and TEG. The study found that TEG provided valuable information about the overall haemostatic status compared to INR in liver surgery patients. It showed a hypercoagulable state in some patients postoperatively, not detected by routine coagulation tests. The study demonstrated that the use of TEG allowed for thromboprophylaxis continuation and facilitated the removal of epidural catheters without requiring blood product transfusion. Additionally, TEG significantly reduced the requirements for blood component transfusion. The study highlighted the importance of TEG in evaluating perioperative coagulation following liver resection and indicated that clinical decisions should be based on TEG parameters rather than PT/INR. The study acknowledges limitations such as the small sample size and the inability to track complications beyond the fifth postoperative day. Nevertheless, the findings support the effectiveness of TEG in assessing coagulation in patients undergoing major liver resections. The paper also references existing literature that emphasizes the poor correlation between TEG values and conventional coagulation tests, the association of liver resections with significant hypercoagulability, and the impact of TEG-guided monitoring on reducing FFP transfusion requirements. Conclusion and Implications In conclusion, the study underscores the significance of TEG in assessing coagulation during major liver resections, presenting a compelling case for its use over conventional coagulation tests. TEG provides valuable insights, aiding in thromboprophylaxis and transfusion strategies, as well as in the management of epidural catheters. The research suggests that TEG could potentially mitigate the risks associated with delayed pharmacological prophylaxis for VTE and the complications of transfusion therapy.
Reference –
Ambulkar, Reshma; Baskar, Vignesh1; Patkar, Shraddha2; Kunte, Aditya2; Agarwal, Vandana1; Solanki, Sohan Lal1; Divatia, Jigeeshu V1. Evaluation of perioperative routine coagulation testing versus thromboelastography for major liver resection – A single-arm, prospective, interventional trial (PORTAL trial). Indian Journal of Anaesthesia 67(12):p 1077-1083, December 2023. | DOI: 10.4103/ija.ija_344_23
Powered by WPeMatico

Researchers have found that mental disorders may be transmitted within adolescent peer networks. This inference comes from a comprehensive population-based study conducted in Finland, examining the potential influence of having classmates with mental disorders on one’s own mental health. The study was published in JAMA Psychiatry and was conducted by Jussi Alho and colleagues.
Previous studies have indicated the possibility of mental disorders being transmitted within social networks. However, these studies often lacked broad epidemiologic evidence that spans a wide range of mental disorders. To address this gap, researchers conducted an extensive analysis of Finnish adolescents, linking demographic, health, and educational data. The objective was to determine whether exposure to classmates diagnosed with mental disorders in the ninth grade is associated with an increased risk of developing a mental disorder later in life.
The study included data on all Finnish citizens born between January 1, 1985, and December 31, 1997. Participants were followed from approximately age 16 (the end of ninth grade) until they were diagnosed with a mental disorder, emigrated, died, or until December 31, 2019. The exposure measured was having one or more classmates diagnosed with a mental disorder in ninth grade. The main outcome was being diagnosed with a mental disorder during the follow-up period.
The study cohort consisted of 713,809 individuals, with a median age of 16.1 years at the start of follow-up, and 50.4% were males.
Of these, 47,433 had a mental disorder diagnosis by ninth grade. Among the remaining 666,376 individuals, 167,227 (25.1%) were diagnosed with a mental disorder during the follow-up period, which encompassed 7.3 million person-years.
Having one diagnosed classmate did not significantly increase the risk (HR, 1.01; 95% CI, 1.00-1.02).
Having more than one diagnosed classmate increased the risk by 5% (HR, 1.05; 95% CI, 1.04-1.06).
The risk was highest during the first year of follow-up:
A 9% increase for one diagnosed classmate (HR, 1.09; 95% CI, 1.04-1.14).
An 18% increase for more than one diagnosed classmate (HR, 1.18; 95% CI, 1.13-1.24).
The risk was particularly pronounced for mood, anxiety, and eating disorders. These associations persisted even after adjusting for parental, school-level, and area-level confounders.
The study suggests that mental disorders might be spread within adolescent peer networks. This potential transmission could be due to several factors, including shared environmental influences, social learning, and emotional contagion. The findings underscore the importance of addressing mental health within school settings and peer groups. This study highlights the potential for mental disorders to be transmitted within adolescent social networks, emphasizing the need for targeted interventions and further research into the mechanisms of such transmission.
Reference:
Alho, J., Gutvilig, M., Niemi, R., Komulainen, K., Böckerman, P., Webb, R. T., Elovainio, M., & Hakulinen, C. (2024). Transmission of mental disorders in adolescent peer networks. JAMA Psychiatry (Chicago, Ill.). https://doi.org/10.1001/jamapsychiatry.2024.1126
Powered by WPeMatico
The FLOW (Evaluate Renal Function with Semaglutide Once Weekly) study is a double-blind, randomised, placebo-controlled international trial comprising 3,533 patients, with a median follow-up period of 3.4 years.
The trial was designed to assess the efficacy and safety of semaglutide, a once-weekly subcutaneous glucagon-like peptide 1 (GLP-1) receptor agonist, in preventing major kidney outcomes, specifically kidney failure, substantial loss of kidney function, and death from kidney or cardiovascular causes, in individuals with type 2 diabetes and chronic kidney disease. Patients either received semaglutide 1.0 mg once weekly or placebo.
Participants who received semaglutide had a 24% risk reduction for the composite primary endpoint, including kidney outcomes and death due to cardiovascular and kidney causes, compared to those who received placebo. This reduction risk was consistent across both kidney-specific and cardiovascular death outcomes.
Secondary endpoints also showed significant improvements with semaglutide. Specifically, the total eGFR slope was 1.16 ml/min/1.73m2/year slower, the risk of major cardiovascular events was decreased by 18%, and the risk of all-cause mortality was reduced by 20%.
This evidence of efficacy, combined with fewer serious adverse events in the semaglutide group, offers hope to millions of patients globally who face the daunting prospect of chronic kidney disease and type 2 diabetes, and their related complications.
Professor Vlado Perkovic, who presented the results for the first time today at the 61st ERA Congress, emphasised the importance of these results, “The use of semaglutide in people with type 2 diabetes and chronic kidney disease can lower the risk of major kidney outcomes and reduce the risk of cardiovascular events, cardiovascular death and all-cause death. These benefits signify a profound clinical impact saving kidneys, hearts and lives, for patients with type 2 diabetes and chronic kidney disease. Additionally, the reassuring safety findings further support the strong potential value of semaglutide in this population.”
Chronic kidney disease affects over 800 million people worldwide and is particularly prevalent among individuals with type 2 diabetes.2 Chronic kidney disease poses a significant risk of kidney failure, cardiovascular events and death,3 highlighting the critical need for research into its prevention and treatment. While current therapies have demonstrated kidney protection and reduced cardiovascular risks, many individuals continue to experience declining kidney function and adverse outcomes. This has sparked a growing interest in exploring new treatments, including GLP-1 receptor agonists.1
Professor Vlado Perkovic further states, “These findings offer great promise in reshaping treatment strategies for individuals at high risk of diabetes-related complications, offering a new avenue for kidney and cardiovascular protection.”
The FLOW trial was overseen by an academic-led Steering Committee, in partnership with the study sponsor, Novo Nordisk, which also managed trial operations. The study is being published today in the New England Journal of Medicine and presented at the 61st ERA Congress in Stockholm, Sweden.
References:
Effects of Semaglutide on Chronic Kidney Disease in Type 2 Diabetes, Perkovic V. et al. (2024). Presented at the ERA Congress 2024.
Kovesdy C. P. (2022). Epidemiology of chronic kidney disease: an update 2022. Kidney international supplements, 12(1), 7–11. https://doi.org/10.1016/j.kisu.2021.11.003
Mann, J. F. E., Buse, J. B., Idorn, T., Leiter, L. A., Pratley, R. E., Rasmussen, S., Vilsbøll, T., Wolthers, B., & Perkovic, V. (2021). Potential kidney protection with liraglutide and semaglutide: Exploratory mediation analysis. Diabetes, obesity & metabolism, 23(9), 2058–2066. https://doi.org/10.1111/dom.14443
Rossing P et al. The rationale, design and baseline data of FLOW, a kidney outcomes trial with once-weekly semaglutide in people with type 2 diabetes and chronic kidney disease. Nephrol Dial Transplant. 2023 Aug 31;38(9):2041-2051. doi: 10.1093/ndt/gfad009. Erratum in: Nephrol Dial Transplant. 2024 Mar 27;39(4):724. PMID: 36651820; PMCID: PMC10469096.
Powered by WPeMatico
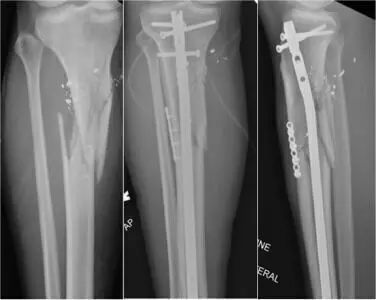
Plate-assisted reduction during intramedullary nailing of tibia shaft fractures may lower risk of nonunion or infection suggests a new study published in the European Journal of Orthopaedic Surgery & Traumatology
The purpose of this meta-analysis is to analyse the literature on plate-assisted reduction during intramedullary nailing of tibial shaft fractures and to compare the rates of infection and nonunion.
The databases Medline, Embase, and Web of Science were searched from inception to February 2022 for literature comparing plate-assisted reduction during intramedullary nailing of extra-articular tibia fractures to standard, closed means of reduction. Data were extracted and pooled in a random effects meta-analysis for the primary outcomes of nonunion and infection risk.
RESULTS
Five comparative studies were identified including 520 total patients, of which 151 underwent tibial nailing with the use of plate-assisted reduction with an average follow-up time of 17.9 months. Approximately two-thirds of patients retained the plate used to assist reduction during intramedullary nailing (102 of 151). Pooled analysis of the infection rates found no significant difference with plate-assisted intramedullary nailing (Risk Ratio [RR] 0.90, 95% CI 0.49-1.65, p = 0.72), and for nonunion rates, there was also no significant difference with plate-assisted intramedullary nailing (Risk Ratio [RR] 0.80, 95% CI 0.40-1.60, p = 0.53).
Plate-assisted reduction during intramedullary nailing of tibia shaft fractures was not associated with an increased risk for nonunion or infection, and can be safely applied as an adjunct for reduction in challenging fracture patterns, without the need for later removal. However, evidence is quite limited and further investigation into the use of provisional plating as a technique is needed as its use in intramedullary nailing continues to expand.
Reference:
Gouveia, Kyle, et al. “Plating as a Reduction Aid Prior to Intramedullary Nailing of Tibia Fractures: a Systematic Review and Meta-analysis.” European Journal of Orthopaedic Surgery & Traumatology : Orthopedie Traumatologie, 2023.
Keywords:
Plate-assisted, reduction, during, intramedullary, nailing, tibia, shaft, fractures, may, lower, risk. nonunion, infection, European Journal of Orthopaedic Surgery & Traumatology : Orthopedie Traumatologie
Powered by WPeMatico
