Early coronary disease, impaired heart function found in asymptomatic people with HIV: Study
A new study found increased coronary vessel wall thickness that was significantly associated with impaired diastolic function in asymptomatic, middle-aged individuals living with HIV. The study was published in Radiology: Cardiothoracic Imaging, a journal of the Radiological Society of North America (RSNA).
According to the World Health Organization, approximately 39 million people were living with HIV at the end of 2022. Since 2010, HIV-related deaths have been reduced by 51%, but HIV continues to be a major global public health issue, claiming 40.4 million lives so far.
As effective therapy drugs increase the life expectancy for people living with HIV, non-AIDS-related health concerns are increasingly common. Recent research has shown that cardiovascular disease is higher in persons living with HIV compared with individuals without HIV, with an estimated 4-fold higher rate of sudden cardiac death compared with the general population.
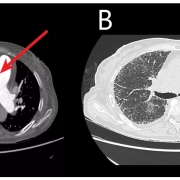
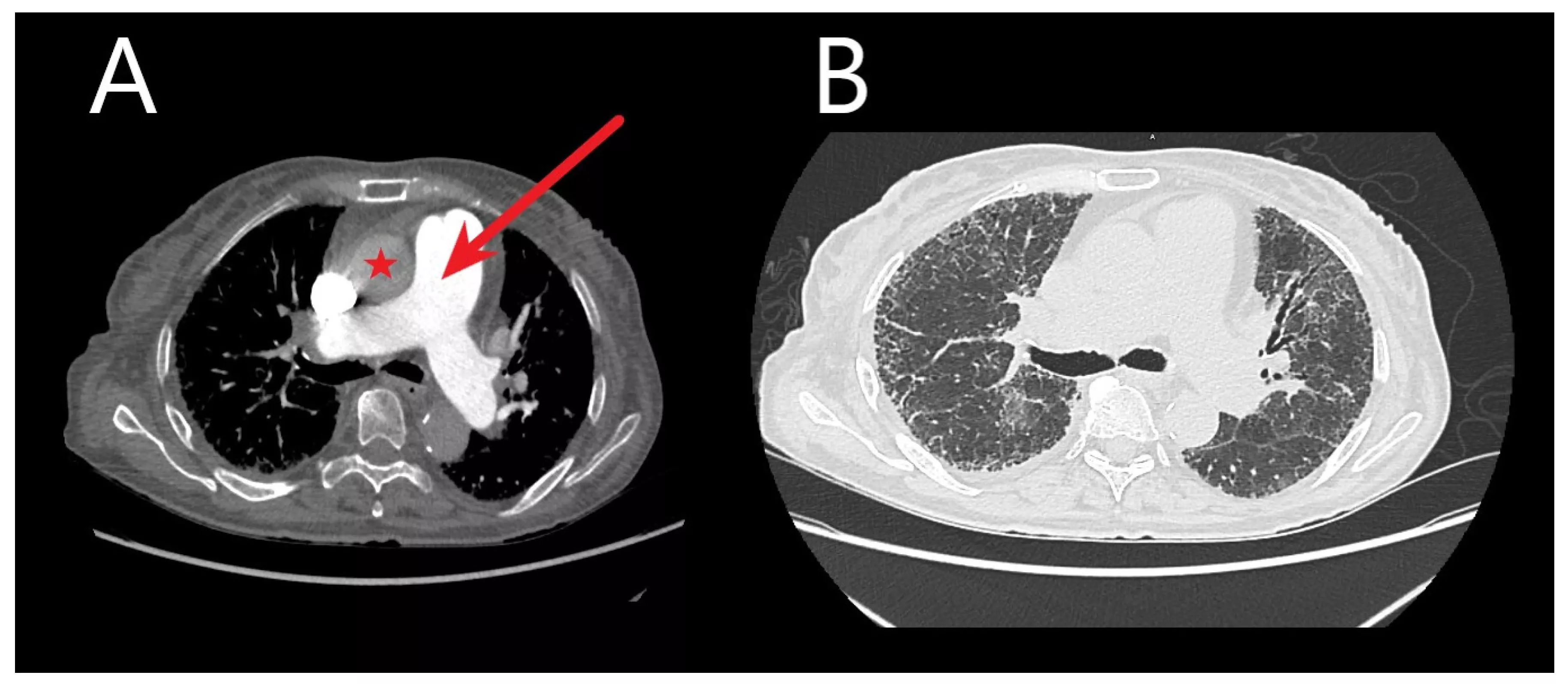
Researchers at the National Institutes of Health (NIH) set out to assess early coronary atherosclerosis burden, and its relation to how the heart is functioning, in people living with HIV who were asymptomatic and had low cardiovascular disease risk. For the study, the researchers recruited 74 adults (mean age of 49 years) living with HIV without known cardiovascular disease and 25 matched healthy controls (mean age of 46 years). Controls were negative for HIV and were required to be healthy with no known significant medical conditions, including coronary disease. All underwent MRI to measure coronary vessel wall thickness and an echocardiogram to assess left ventricular function.
“Prior studies have shown cardiovascular disease in persons living with HIV, however not at such an early stage,” said senior author Ahmed M. Gharib, M.D., senior clinical investigator, and director of the Biomedical and Metabolic Imaging Branch, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), in Bethesda, Maryland.
The HIV and control groups were matched by corresponding age, sex and race. A detailed medical history, physical examination and laboratory tests were obtained from participants, including detailed review of any exposure to antiretroviral therapy-the combination of several antiretroviral medicines used to slow the rate at which HIV makes copies of itself (multiplies) in the body-and traditional cardiovascular disease risk factors. Each participant had lab tests that included the following: fasting lipid panel, T-cell counts and HIV viral load.
The results showed increased coronary vessel wall thickness in the HIV group, compared to controls. The increased coronary artery vessel wall thickness was independently associated with elevated left ventricular mass index and impaired diastolic function.
Coronary artery vessel wall thickness was also associated with the duration of exposure to didanosine, one of the medications used in combination with other drugs for the treatment of HIV.
“The ability to detect early coronary artery disease in persons living with HIV and potentially prevent detrimental effects on the heart muscle is important,” said lead author Khaled Z. Abd-Elmoniem, Ph.D., M.H.S., staff scientist in NIDDK’s Biomedical and Metabolic Imaging Branch. “This research shows the impact of HIV on developing subtle subclinical coronary artery disease and its effects on heart function.”
The researchers emphasized that early identification of subclinical cardiovascular disease in young HIV patients is an urgent necessity, potentially opening avenues for more effective intervention and disease management.
Reference:
Khaled Z. Abd-Elmoniem, Hadjira Ishaq, Julia Purdy, Jatin Matta, Ahmed Hamimi, Hwaida Hannoush, Colleen Hadigan , Ahmed M. Gharib, Association of Coronary Wall Thickening and Diminished Diastolic Function in Asymptomatic, Low Cardiovascular Disease–Risk Persons Living with HIV, Radiology Cardiothoracic Imaging, https://doi.org/10.1148/ryct.230102.
Powered by WPeMatico