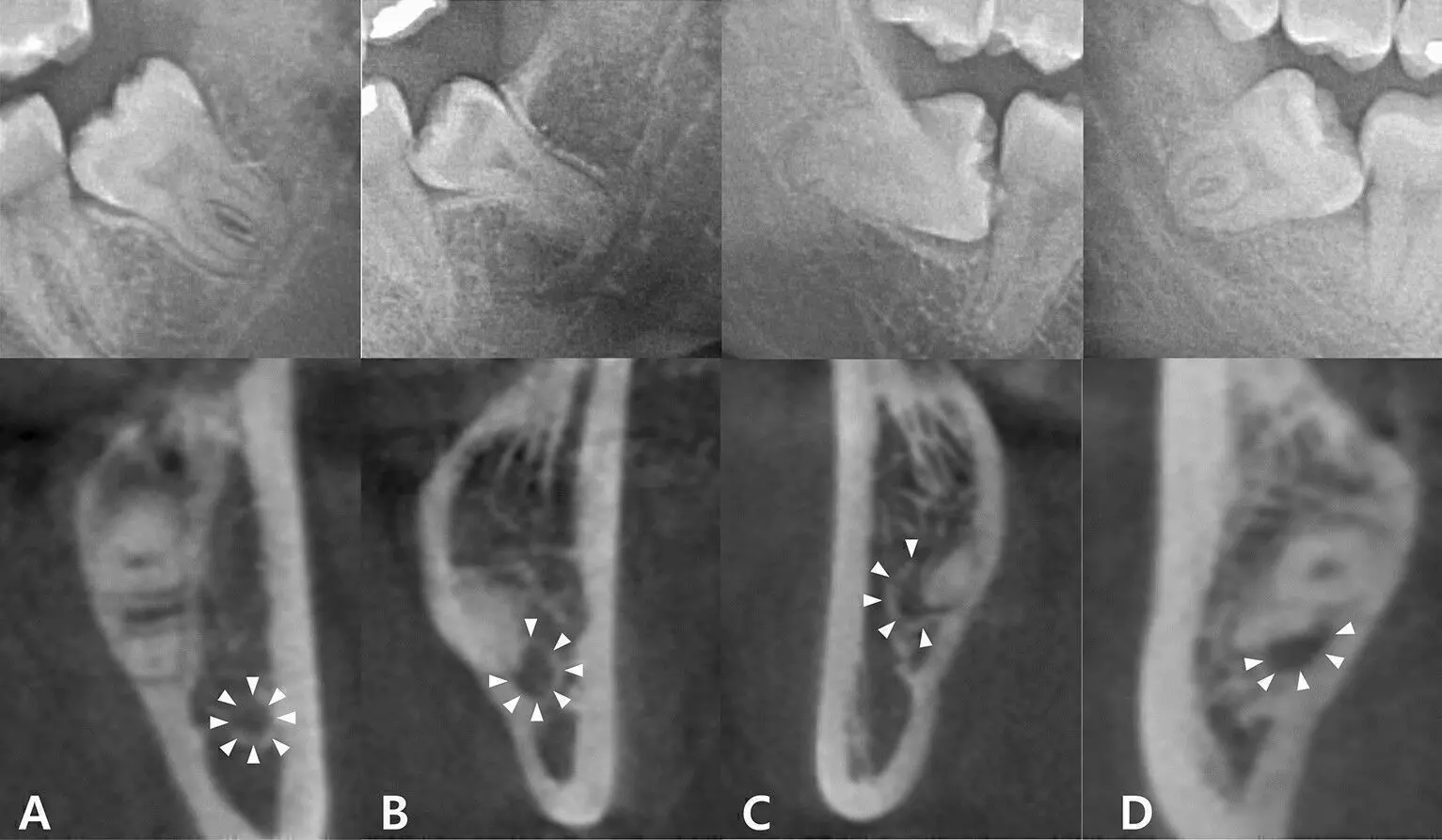
New Delhi: The National Consumer Disputes Redressal Commission (NCDRC) recently held a surgeon and a hospital liable for not taking consent for the removal of a 6cm sciatica nerve of a patient, along with not recording its removal in post-operative notes, informing about it to the patient and providing requisite counselling.
Holding them liable for deficiency in service, the Apex Consumer Court directed them to pay Rs 5 lakh lump sum compensation to the patient. Earlier, the State Commission had also ordered them to reimburse the cost of treatment incurred after the surgery. However, the top consumer court noted that the doctor and hospital defended the requirement of cutting the sciatica nerve to save the patient.
“…while the requirement of cutting sciatica nerve has been adequately defended, at the same time, not obtaining the consent and not recording in the post operation notes as well as informing him and guiding him with respect to handling such conditions at the time of discharge certainly constitutes negligence on part of OP-1 and 2,” it held.
The history of the case goes back to 2012 when the patient consulted Dr. Kokne, a surgeon, at the treating hospital. It was alleged the doctor recommended an operation to remove a tumour in the patient’s thigh, assuring him that he would regain proper mobility afterwards. Accordingly, the complainant’s wife gave consent for the surgery and the operation was performed by the surgeon, who excised the tumour from the patient’s left thigh.
However, the patient alleged that during the procedure, the doctor negligently severed the 6 CM sciatic nerve, the primary nerve of the left leg. Due to this, the patient lost sensation and mobility in his left and became unable to walk unaided. Further, it was also alleged that the doctor failed to mention the severance of the sciatic nerve in the post-operative notes.
Thereafter, two days after the surgery, Dr Ravi, a pathologist provided a test report revealing that the complainant was suffering from cancer (“Atypical Lipoma favoring well Differentiated Liposarcoma”). Consequently, the patient was referred to Gupte Cancer Clinic, where cancer treatment was initiated, including chemotherapy.
The patient alleged that the Clinic erroneously reported improvement in sensation in his left leg. He also submitted that he incurred Rs 3 lakh as expenses for the chemotherapy. Subsequently, due to mobility issues, he took medical advice from Christian Medical College, where a re-examination of the excised tumour tissue revealed no definitive evidence of cancer.
It was mentioned in the report, “In CMC, review of previous histology showed no definition evidence of a high-risk grade sarcoma (however atypical lipomatous tumour cannot be excluded).”
Therefore, he was advised to undergo another operation. Following this, the patient sought a second opinion from the doctors at Meditrina Institute of Medical Science, Nagpur, where the post-operative injury to the left sciatic nerve, resulting in a left foot drop, was diagnosed. Consequently, a surgery was performed to repair the severed nerve, incurring Rs 3,75,000 for tests, medicines, and hospital fees, and Rs 4,25,000 for operation.
Alleging that due to the negligence of the treating surgeon and pathologist, coupled with the erroneous communication of cancer diagnosis and further treatment by the Cancer Clinic, the complainant sent legal notices to the parties and sought Rs 18,30,000 compensation. When the doctors and hospital refused to pay the compensation, the complainant filed before the District Forum, seeking compensation with interest and litigation costs.
On the other hand, the doctors and hospital refuted all allegations and contended that the hospital appointed Dr. Kokne as a Surgeon at their hospital. The complainant underwent tumour testing at a hospital in Nagpur through sonography, where a doctor advised him to undergo tumour removal surgery. It was also submitted that the patient had been living with a tumour for 15-20 years and the family members were informed that both the tumour and its surrounding area needed removal.
It was submitted that during the operation on 07.08.2012, the doctor discovered that the tumour was cancerous and had spread to the surrounding area. They further claimed that to prevent endangering the patient’s life, both the tumour and the affected surrounding area were excised. Allegedly, the patient’s relatives were informed of this situation and the tumour was later sent to Dr. Ravi for testing, after which it was confirmed to be cancerous.
The doctor claimed that he followed the principle of “life over limb” in medical jurisprudence, prioritizing the patient’s life over a particular body part and denied the allegations of any medical negligence. Further, the doctor stated that the complainant did not adhere to the instructions and the prescribed post-operative medication and instead sought chemotherapy based on the cancer diagnosis.
Meanwhile, the pathologist and the cancer clinic refuted these allegations and contended that since the surgeon and hospital conducted the tumour operation, it was not appropriate for them to respond to the contentions. They also submitted that the necessity of the tumour removal due to its size and the entanglement of the left sciatic nerve with the tumour, led to its natural severance during the operation. They refuted the claim that the Complainant’s left leg became non-functional due to this.
The pathologist further referred to the report which indicated Atypical Lipoma Favors well differentiated Liposarcoma” due to observed tissue changes. He submitted that even though he advised further testing through “Immunohistology Chemistry”, the patient did not undergo the same. He also referred to the similarity between Dr Ravi and Vellore’s reports with a slight difference in wording, both indicated diagnosis of “Atypical Lipomatous tumour”. Vellore’s experts also recommended radiotherapy. Regarding nerve entanglement with the tumour, it was argued that, it is customary to severe the nerve to the extent needed, with the possibility of re-establishing it by a simple operation. They asserted that they did not provide negligent services.
While considering the matter, the District Commission held that there was no negligence and accordingly it dismissed the complaint. However, the State Commission held that the operating surgeon should have obtained informed consent and also should have noticed the injury to the Sciatic Nerve and accordingly the necessary information should have been given to the patient as well as the relatives.
However, it held that the pathologist and the cancer clinic performed their duties in accordance with the reports which they received and therefore, there was no negligence or deficiency on their part in providing treatment to the complainant. Accordingly, the State Commission had partly allowed the complaint with a cost quantified to Rs 25,000 to be paid by the surgeon and the hospital to the complainant. They were also directed to reimburse the treatment expenditure of Rs 3,75,000 at Christian Medical College, Vellore and the treatment expenditure at Meditrina Institute of Medical Science Nagpur Rs 4,25,000 along with interest. Further, they were directed to pay Rs 5 lakh as compensation towards physical and mental harassment and loss of income.
However, the order of the State Commission was challenged before the Apex Consumer Court. While considering the matter, the NCDRC bench noted that the complainant had a tumour in his left thigh for more than 15 years. The surgeon advised surgery to remove the tumour. Accordingly, the surgeon excised the tumour along with surrounding tissues, as well as a part of his main sciatic nerve. This resulted in loss of sensation and rendered him unable to walk without assistance. Subsequently, based on the pathological examination of the tissue, the Complainant was diagnosed with cancer leading to chemotherapy treatment.
The NCDRC noted that further examination revealed that there was no definite evidence of cancer in the tumour tissue and the patient underwent another surgery to repair the damaged nerve, incurring significant expenses for medical treatment and surgery. Therefore, the dispute is centred on the adequacy of informed consent, the standard of care during surgery, the accuracy of diagnosis and the appropriateness of the treatment provided, noted the top consumer court.
“The material difference between the aspects of medical care lies in the degree of passivity on the part of the patient. The diagnosis and treatment are in the domain of doctor, and the patient is a passive participant. When advice is being given to the patient, the patient assumes an active role. Then doctor’s function is to empower and enable the patient to make a decision by giving him relevant, sufficient and material information. The patient must make choices and decisions. The patient must be informed about the options for treatment, its consequences, risks and benefits. Why doctor thinks particular treatment necessary and appropriate for the patient. The prognosis and what may happen if treatment is delayed or not given,” noted the consumer court.
“Failing to furnish correct sufficient information when obtaining consent may be a breach of duty of care. It amounts to negligence, failure to inform the patient. The patient must be given a reasonable amount of time to consider the information to make a decision. The allowing of cooling off period is for the purpose to give time to think over the decision or take advice so that patient does not feel pressurized or rushed to sign. On the day of surgery, the patient may be under strain, mental stress or under influence of the pre-procedure drugs which may hamper his decision-making ability. The doctor performing any procedure must obtain the patient’s consent; no one else can consent on behalf of the competent adult. The consent should be properly documented and preferably witnessed as such consent is legally more acceptable. The video recording of the informed consent process may also be done with a prior consent of the patient for the same,” it further observed.
The Commission noted that the main assertion of the complainant was that the removal of the sciatica nerve was neither explained to him nor his wife prior to surgery or during the surgery. Further, it was allegedly not reflected in the operation notes as well.
“There is no evidence on record to indicate that consent for removal of sciatic nerve was taken. The post operation record also does not have mention of the same. There is also no record to indicate that the Complainant was even informed of to such removal and was given necessary guidelines to dealing with such condition and process for restoration. Therefore, while the requirement of cutting sciatica nerve has been adequately defended, at the same time, not obtaining the consent and not recording in the post operation notes as well as informing him and guiding him with respect to handling such conditions at the time of discharge certainly constitutes negligence on part of OP-1 and 2. The Complainant subsequently underwent surgery in Meditrina Institute and got the sciatica nerve restored. Therefore, it deserves to be compensated,” NCDRC noted at this outset.
“In any case with respect to pathological examination, diagnosis and treatment of cancer, OP-1 and 2 have no role. Also no liability has been attributed in this regard against OP-3 and 4,” said the Consumer Court.
Holding the treating surgeon liable, NCDRC noted,
“In view of the foregoing with respect to allegation of medical negligence, the liability of OP-1 and 2 is with respect to removal of 6 cm sciatica nerve, without taking consent, not recording in the post operation notes, not notifying the Complainant even thereafter and not giving him requisite counseling in handling the situation as part of discharge notes. These failures do not align with what a reasonable medical professional would do in similar circumstances. Therefore, OP-1 and 2 are liable to this extent.”
Modifying the State Commission’s order, the Apex Commission observed, “The Petitioners/OP-1&2 are jointly and severally directed to pay a lump sum of Rs.5,00,000/- to the Complainant on account of deficiency in service in not taking consent for removal of 6 cm sciatica nerve without taking consent, not recording its removal in post operation notes, not notifying the Complainant even thereafter and not giving him the requisite counseling as part of discharge notes, mental agony and harassment, loss of income and litigation costs. This amount shall be paid within one month from the date of this order. In the event of delay beyond the said period of one month, the simple interest applicable for such extended period shall be @ 12% per annum, till realization.”
To view the order, click on the link below:
https://medicaldialogues.in/pdf_upload/ncdrc-5-lakh-compensation-236748.pdf
Also Read: Monitoring lapse post Septoplasty leads to patient’s death: ENT surgeon, Anaesthesiologist, Hospital slapped Rs 30 lakh compensation