Respiratory syncytial virus has increased frequency of transplacental transmission in Pregnant Women: Study
A recent comprehensive study illuminated the transmission of respiratory viral infections from pregnant women to their offspring. This research uncovered significant disparities between the respiratory syncytial virus (RSV) and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The key findings were published in the recent issue of American Journal of Respiratory and Critical Care Medicine.
This prospective cohort study was conducted to discern the frequencies of transplacental transmission of these viruses, analyze concentrations of inflammatory mediators in maternal and fetal blood and assess clinical consequences. The finding, spanning from October 2020 to June 2022, were published in a recent medical journal.
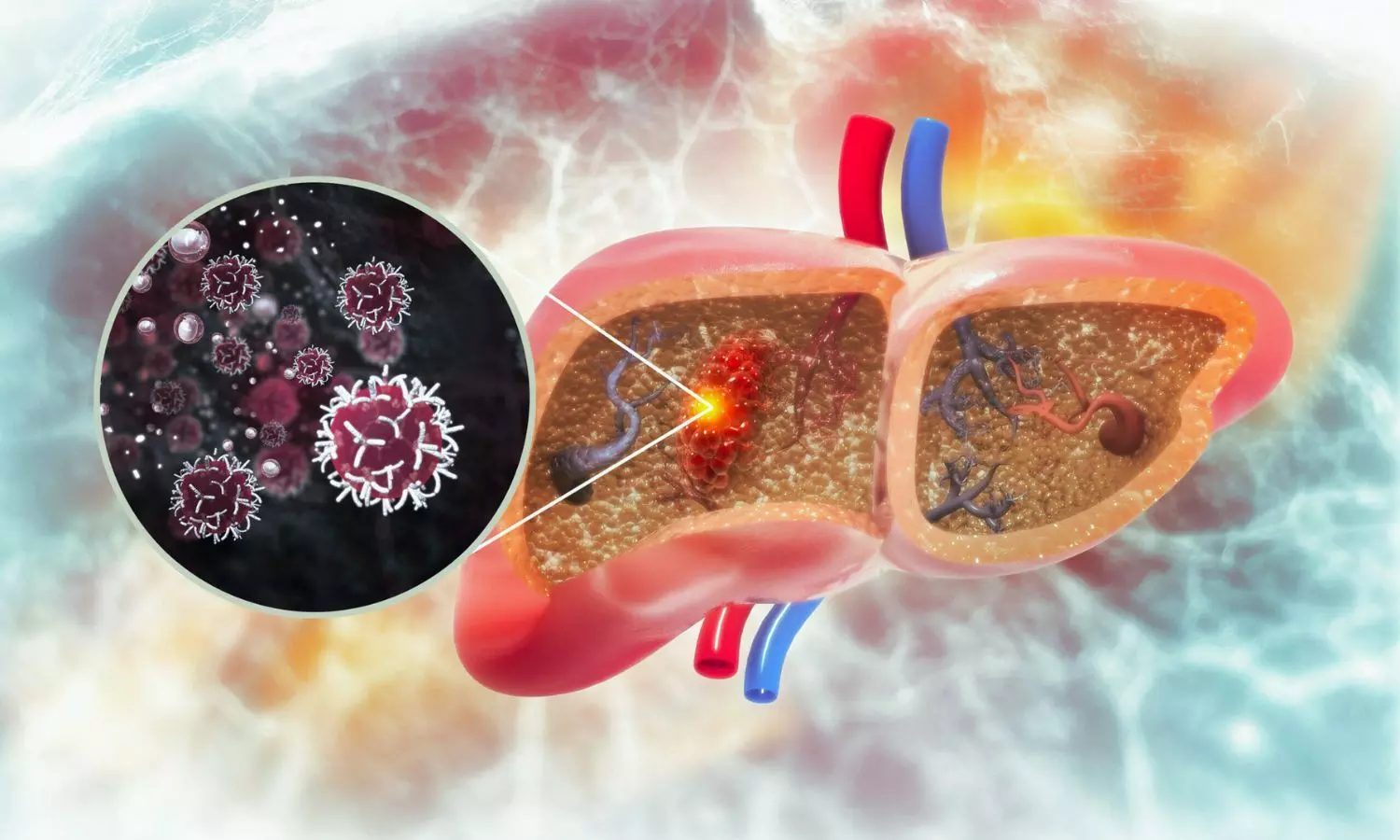
The study included a total of 103 mother–baby dyads and utilized droplet digital PCR to test blood mononuclear cells. Also, the results revealed that twice as many newborns were vertically infected with RSV when compared to SARS-CoV-2 where the rates stood at 25.2% and 11.9%, respectively. This sharp contrast underlines the differing transmission dynamics of these respiratory viruses during pregnancy.
Further analysis through multiplex ELISA showed significantly increased concentrations of various inflammatory cytokines and chemokines in both maternal and cord blood from newborns with evidence of viral exposure in utero. This suggests a strong link between prenatal infection and fetal inflammation with the distinct expression profiles contingent upon the virus type.
Moreover, this study unveiled consequential impacts on newborn health. Babies exposed to these viruses in utero experienced lower birth weights and exhibited compromised postnatal weight growth. These effects signify the potential pathological consequences of virus-induced inflammation on neonatal health, manifesting within the first days of life.
The study emphasized the importance of these findings in understanding the intricate dynamics of vertical transmission and its implications for maternal and neonatal health. This research elucidates the complexities of prenatal viral infections and the need for higher vigilance in managing respiratory illnesses during pregnancy
This study serves as a crucial milestone in elucidating the nuances of vertical transmission. Further research and clinical insights is mandated that could better help the healthcare professionals customize strategies to safeguard the health and well-being of both pregnant women and their offspring.
Research:
Trinh, I. V., Desai, S. P., Ley, S. H., Mo, Z., Satou, R., Pridjian, G. C., Longo, S. A., Shaffer, J. G., Robinson, J. E., Norton, E. B., & Piedimonte, G. (2024). Prenatal Infection by Respiratory Viruses Is Associated with Immunoinflammatory Responses in the Fetus. In American Journal of Respiratory and Critical Care Medicine (Vol. 209, Issue 6, pp. 693–702). American Thoracic Society. https://doi.org/10.1164/rccm.202308-1461oc
Powered by WPeMatico