Migraines and persistent vasomotor symptoms jointly associated with greater risk for CVD, stroke: Study

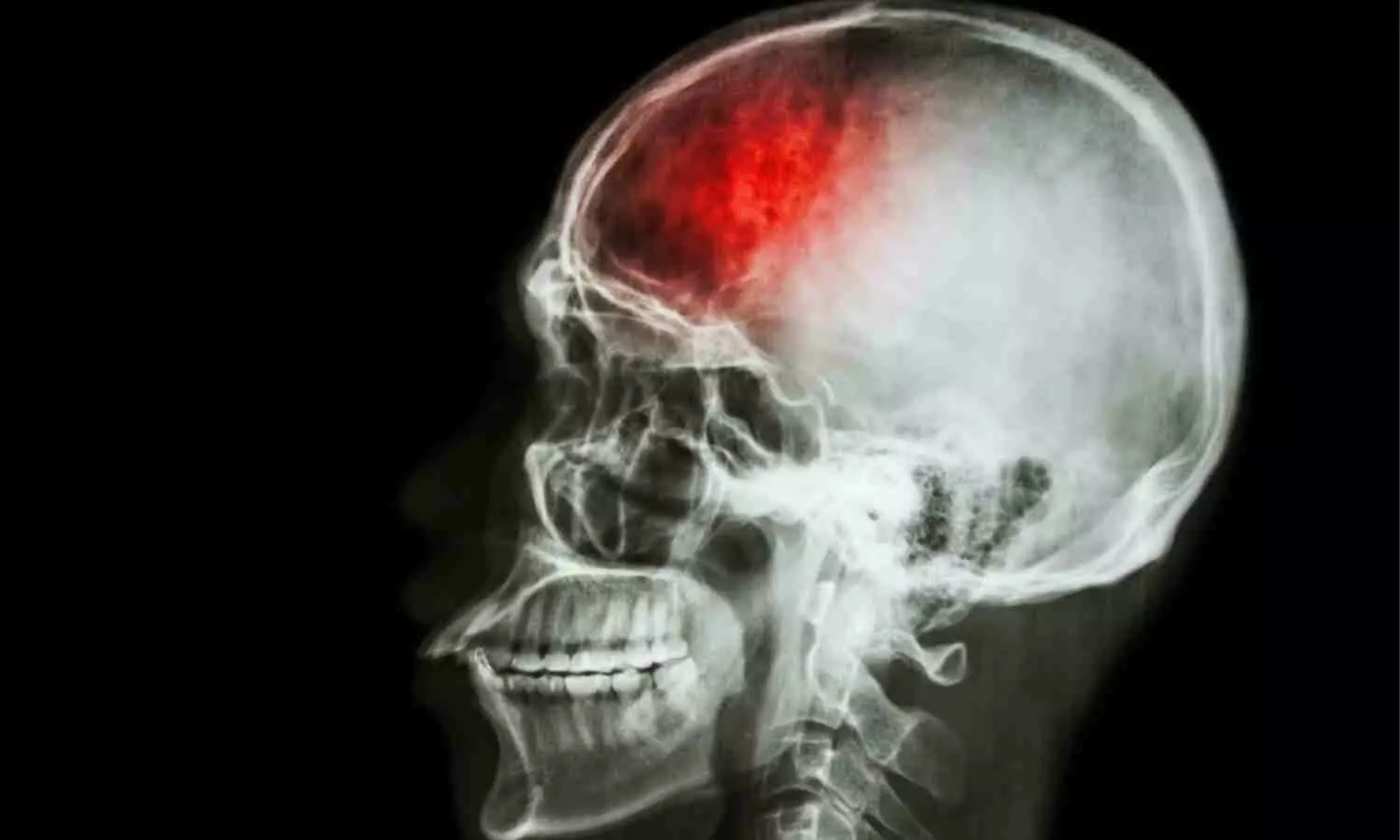
USA: A recent study published in Menopause has revealed a joint association of migraines and persistent vasomotor symptoms (VMS) with a greater risk of stroke and cardiovascular diseases, although the risk attenuates with adjustment for traditional CVD risk factors.
Catherine Kim, Departments of Medicine, Obstetrics and Gynecology, and Epidemiology, University of Michigan, Ann Arbor, MI, and colleagues conducted the study to examine whether vasomotor symptoms and migraine headaches, hypothesized to be vasoactive conditions, are associated with greater risk for cardiovascular disease events including strokes.
For this purpose, the researchers performed a secondary data analysis of a subset of women (n = 1,954) in a population-based cohort, the CARDIA study, which began data collection at 18 to 30 y of age. They examined whether migraine headaches and VMS trajectories (characterized as minimal, increasing, and persistent) at CARDIA year 15 examination were linked with a higher risk of stroke (both ischemic and hemorrhagic) and CVD events using Cox proportional hazards regression models and adjustment for traditional CVD risk factors (cigarette use, age, and levels of systolic and diastolic blood pressure, high- and low-density cholesterol, fasting glucose, and triglycerides) and reproductive factors.
Based on the study, the researchers reported the following findings:
- Among women with minimal VMS (n = 835), increasing VMS (n = 521), and persistent VMS (n = 598), there were 81 incident CVD events, including 42 strokes.
- Women with histories of migraine and persistent VMS had a greater risk of CVD (hazard ratio [HR], 2.25) after adjustment for age, race, estrogen use, oophorectomy, and hysterectomy compared with women without migraine histories and with minimal/increasing VMS.
- After adjustment for CVD risk factors, these associations were attenuated (HR, 1.51).
- Women with histories of migraine and persistent VMS had a greater risk of stroke (HR, 3.15), but these associations were attenuated after adjustment for CVD risk factors (HR, 1.70).
“Migraines and persistent vasomotor symptoms jointly associate with a greater risk for cardiovascular disease and stroke, although the risk is attenuated with adjustment for traditional CVD risk factors,” the researchers wrote.
Reference:
Kim, Catherine MD, MPH1; Schreiner, Pamela J. PhD2; Yin, Zhe MS3; Whitney, Rachael PhD4; Sidney, Stephen MD, MPH5; Ebong, Imo MD6; Levine, Deborah A. MD, MPH4. Migraines, vasomotor symptoms, and cardiovascular disease in the Coronary Artery Risk Development in Young Adults study. Menopause ():10.1097/GME.0000000000002311, February 13, 2024. | DOI: 10.1097/GME.0000000000002311
Powered by WPeMatico