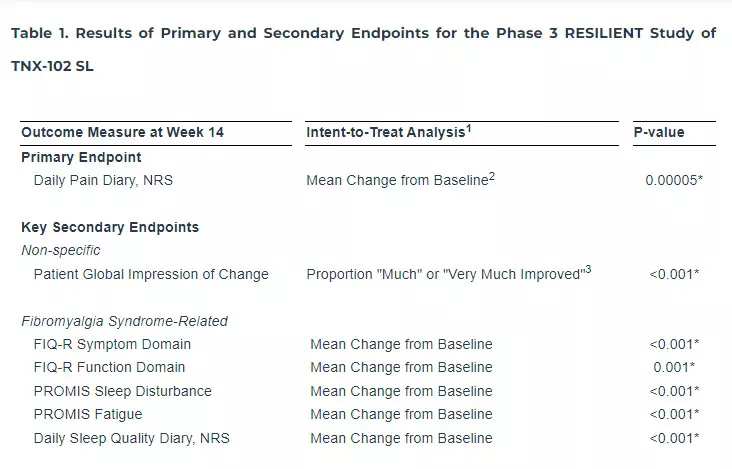
Tonix Pharmaceuticals Holding Corp. (Nasdaq: TNXP) (Tonix or the Company), a biopharmaceutical company with marketed products and a pipeline of development candidates, today announced that the Phase 3 RESILIENT study evaluating TNX-102 SL (cyclobenzaprine HCl sublingual tablets) met its pre-specified primary endpoint in the second of two positive Phase 3 clinical trials, significantly reducing daily pain compared to placebo (p=0.00005) in participants with fibromyalgia (Table 1). Statistically significant and clinically meaningful results were also seen in all key secondary endpoints related to improving sleep quality, reducing fatigue, and improving overall fibromyalgia symptoms and function. Additionally, as it relates to improving daily pain, treatment with TNX-102 SL showed a robust and clinically meaningful analgesic effect size of 0.38, with rapid onset of action, separating from placebo for each week of the study. TNX-102 SL was well tolerated with an adverse event profile comparable to prior studies, and no new safety signals were observed. Tonix plans to submit a New Drug Application (NDA) to the U.S. Food and Drug Administration (FDA) in the second half of 2024 for TNX-102 SL for the management of fibromyalgia. An estimated 6 million to 12 million U.S. adults are living with fibromyalgia, the majority of whom are women.
TNX-102 SL is a tablet formulation containing 2.8 mg cyclobenzaprine HCl and is a novel, centrally-acting, non-opioid analgesic, designed to be taken once daily at bedtime for the management of fibromyalgia. RESILIENT was a 14-week randomized, double-blind, placebo-controlled trial of TNX-102 SL 5.6 mg, in which 457 participants with fibromyalgia were randomized in a 1:1 ratio to TNX-102 SL or placebo across 33 sites in the U.S. All participants received one 2.8 mg tablet of TNX-102 SL (2.8 mg) or placebo for the first 2 weeks, which was increased to two 2.8 mg tablets of TNX-102 SL (5.6 mg) or placebo for the remaining 12 weeks.
In December 2020, Tonix reported positive results from the first Phase 3 RELIEF study of TNX-102 SL 5.6 mg for the management of fibromyalgia. The RELIEF study met its pre-specified primary endpoint, significantly reducing daily pain compared to placebo (p=0.010) in participants with fibromyalgia, and showing activity in key secondary endpoints.
“We believe that the positive results of RESILIENT and RELIEF show that fibromyalgia can be successfully treated by TNX-102 SL 5.6 mg and may provide the opportunity for Tonix to have the first FDA-approved drug for fibromyalgia in more than a decade,” said Seth Lederman, M.D., President and Chief Executive Officer of Tonix Pharmaceuticals. “We are now an important step closer to bringing a new, first-line treatment to fibromyalgia patients that offers broad symptom relief and favorable tolerability for chronic use and adherence. We believe that we are well positioned to submit an NDA to the FDA under the 505(b)(2) regulatory approval pathway in the second half of 2024, and are on track to supply the U.S. market upon FDA approval.”
Abbreviations: FIQ-R = Fibromyalgia Impact Questionnaire – Revised; NRS = Numeric Rating Scale; PROMIS = Patient-Reported Outcomes Measurement Information System
*Statistically significant; to control for overall type 1 error, a pre-specified, serial gatekeeping procedure was utilized.
1Analysis by mixed model repeated measures with multiple imputation unless otherwise indicated.
2Primary endpoint analysis for FDA approvals of Cymbalta® and Lyrica® in fibromyalgia.
3Pearson’s chi-squared test responder analysis, with missing data considered non-responders
“These data are terrific news for patients with fibromyalgia,” said Daniel J. Clauw, M.D., Professor of Anesthesiology, Medicine and Psychiatry at the University of Michigan. “Despite approved medications, there remains a need for new treatment options to better address the quality of life impacts many fibromyalgia patients experience on a chronic basis. TNX-102 SL is a non-opioid, centrally-acting analgesic, the active ingredient of which has a known, favorable safety profile from decades of use. The fact that cyclobenzaprine was also beneficial in many other key symptom domains, including sleep quality, sleep disturbance and fatigue, will be appreciated by fibromyalgia patients that struggle with not just pain but multiple other symptoms.”
“These positive data from RESILIENT and previously with RELIEF, with remarkable separation from placebo on pain, sleep, and fatigue, add support to TNX-102 SL’s proposed mechanism of improving sleep quality to improve the syndromal effects of fibromyalgia,” commented Gregory Sullivan, M.D., Chief Medical Officer of Tonix Pharmaceuticals. “The sublingual formulation of TNX-102 SL, which uses our proprietary Protectic® and Angstro® technologies, is integral to our treatment paradigm. These technologies enable transmucosal delivery of cyclobenzaprine with distinctive pharmacokinetic properties that include rapid absorption after dosing and bypass of first-pass hepatic metabolism. I would like to thank the RESILIENT study participants and their families and caregivers, as well as the investigators and their hard-working staff who all made this a highly successful trial.”
Summary of Topline Results of the RESILIENT Study
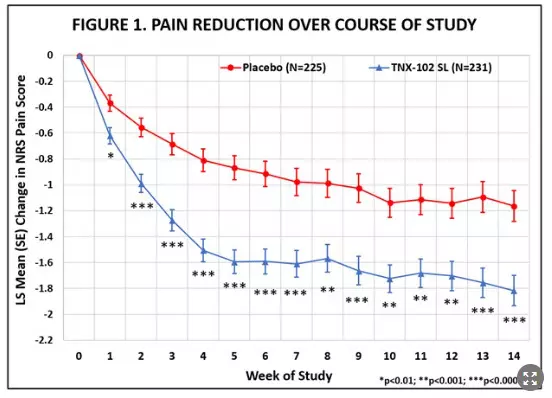
The RESILIENT study achieved statistical significance on the pre-specified primary efficacy endpoint: change from baseline in the weekly average of daily diary pain severity numerical rating scale (NRS) scores for TNX-102 SL 5.6 mg (LS mean [SE]: -1.8 [0.12] units) versus placebo (-1.2 [0.12] units), analyzed by mixed model repeated measures with multiple imputation (LS mean [SE] difference: -0.7 [0.16] units, p=0.00005, Table 1). In addition, all pre-specified sensitivity analyses of the primary endpoint were statistically significant (p<0.001). Figure 1 shows reduction in pain across all weeks of the 14-week study, with nominal p<0.01 for every week. Note the rapid onset of action with separation from placebo at Week 1 was sustained throughout all weeks of dosing.
Abbreviations: LS = least squares; NRS = numerical rating scale; SE = standard error
The statistically significant improvement in pain is further substantiated when diary pain was analyzed by another standard statistical approach, a 30 percent responder analysis, with 45.9% on active and 27.1% on placebo having a 30 percent or greater reduction in pain (Pearson Chi-Squared Test; difference in proportions [95% CI]: 18.8% [10.1%, 27.4%]; nominal p<0.001).
TNX-102 SL showed statistical significance (p≤0.001) on all six pre-specified key secondary efficacy outcome measures (Table 1).
Consistent with the proposed mechanism that TNX-102 SL acts in fibromyalgia through improving sleep quality, TNX-102 SL showed statistically significant improvement of sleep by two main measures. For the daily diary sleep quality ratings, improvement in sleep quality for TNX-102 SL (-1.8 [0.12] units) was significantly greater than that of placebo (-1.2 [0.12] units; LS mean [SE] difference from placebo: -0.6 [0.17] units; p<0.001). For the PROMIS Sleep Disturbance instrument, TNX-102 SL also demonstrated significantly greater improvement over placebo on T-scores (LS mean [SE] difference from placebo: -4.2 [0.79] units; p<0.001). Fatigue is another cardinal symptom of fibromyalgia and has a major impact on quality of life. TNX-102 SL showed significant improvement over placebo on the PROMIS Fatigue instrument T-scores (-3.0 [0.77] units; p<0.001).
The Fibromyalgia Impact Questionnaire – Revised (FIQ-R) is a 21-item self-rated instrument that assesses level of function, overall impact, and symptoms due to fibromyalgia, and the symptoms and function domains were key secondary endpoints in RESILENT. At Week 14 on the FIQ-R Symptoms domain, there was significantly greater improvement with TNX-102 SL than with placebo (LS mean [SE] difference from placebo: -7.7 [1.62], p<0.001). Similarly, TNX-102 SL resulted in greater improvement on FIQ-R Function (LS mean [SE] difference from placebo: -5.4 [1.66], p=0.001). Although not a key secondary efficacy endpoint, TNX-102 SL also separated from placebo on the FIQ-R Impact domain (nominal p=0.001). These results, along with the robust effects on improving sleep and fatigue, suggests broad symptomatic coverage of the syndrome of fibromyalgia.
Safety Results of the Phase 3 RESILIENT Study
In the RESILIENT study, TNX-102 SL was well tolerated and consistent with prior trials, with no new safety signals observed. Among participants randomized to the TNX-102 SL and placebo arms, 81.0% and 79.2%, respectively, completed the 14-week dosing period. As expected based on prior TNX-102 SL studies, administration site reactions were the most commonly reported adverse events and were higher in the TNX-102 SL treatment group (Table 2). Hypoaesthesia oral and paraesthesia oral, or tongue and mouth numbness or tingling, product taste abnormal (typically a bitter aftertaste upon dosing), and tongue discomfort were local effects nearly always temporally related to dose administration and transiently expressed (<60 minutes) in most occurrences. The only treatment-emergent adverse events that occurred at a rate of 3.0% or greater in either arm were these four oral adverse events, along with COVID-19, somnolence, and headache (Table 2). Adverse events resulted in premature study discontinuation in 6.1% of those who received TNX-102 SL compared with 3.5% of placebo recipients. There were a total of seven serious adverse events in five patients, five of which were experienced by three patients in the placebo arm, and two of which were in the TNX-102 SL arm. Of the two in the TNX-102 SL arm, one was renal cancer, deemed unrelated to study drug, and the other was acute pancreatitis with onset 14 days after dosing was completed and reported as possibly related to study drug.

The Changes in Sexual Functioning Questionnaire short form (CSFQ-14) served as a safety measure for assessing potential adverse effects on sexual functioning. In females, the total score on the CSFQ-14 at Week 14 improved (indicating better sexual functioning) in the TNX-102 SL group compared with placebo (nominal p=0.010 by analysis of covariance). This potentially indicates an important tolerability advantage over pharmacotherapeutics which potently inhibit reuptake of serotonin. The low percentage of males in the safety population (<5%) did not allow meaningful analysis of the CSFQ-14 data.
About the Phase 3 RESILIENT Study
The RESILIENT study is a double-blind, randomized, placebo-controlled trial designed to evaluate the efficacy and safety of TNX-102 SL (cyclobenzaprine HCl sublingual tablets) in the management of fibromyalgia. The two-arm trial randomized 457 participants in the U.S. across 33 sites. The first two weeks of treatment consist of a run-in period in which participants start on TNX-102 SL 2.8 mg (1 tablet) or placebo. Thereafter, all participants increase their dose to TNX-102 SL 5.6 mg (2 x 2.8 mg tablets) or two placebo tablets for the remaining 12 weeks. The primary endpoint is the daily diary pain severity score change (TNX-102 SL 5.6 mg vs. placebo) from baseline to Week 14 (using the weekly averages of the daily numerical rating scale scores), analyzed by mixed model repeated measures with multiple imputation.