Pfizer Marstacimab regulatory submissions for treatment of Hemophilia A and B accepted by USFDA, EMA

New York: Pfizer Inc. has announced that the U.S. Food and Drug Administration (FDA) has accepted the company’s Biologics License Application (BLA) for its anti-tissue factor pathway inhibitor (anti-TFPI) candidate marstacimab for individuals living with hemophilia A or hemophilia B without inhibitors to Factor VIII (FVIII) or Factor IX (FIX). The European marketing authorization application (MAA) for marstacimab also passed validation and is currently under review by the European Medicines Agency (EMA).
The FDA has set a Prescription Drug User Fee Act (PDUFA) action date in the fourth quarter of 2024, and a decision from the European Commission is anticipated by the first quarter of 2025. “If approved in the U.S. and EU, marstacimab is expected to become the first once-weekly subcutaneous treatment for people living with haemophilia B and the first treatment administered as a flat dose for people living with haemophilia A or B,” the Company stated in a release.
“Marstacimab has demonstrated that it may be an efficacious treatment option with once-weekly, subcutaneous flat-dose administration via an auto-injector pen, for appropriate patients, if approved. This is critical as intravenous infusions are typically required for people living with these diseases today,” said James Rusnak, M.D., Ph.D., Senior Vice President, Chief Development Officer, Internal Medicine and Infectious Diseases, Research and Development, Pfizer. “We look forward to progressing the review of this novel therapy with the FDA, EMA, and global regulatory authorities to bring this important medicine to patients globally.”
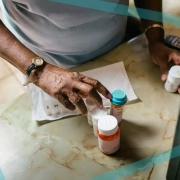
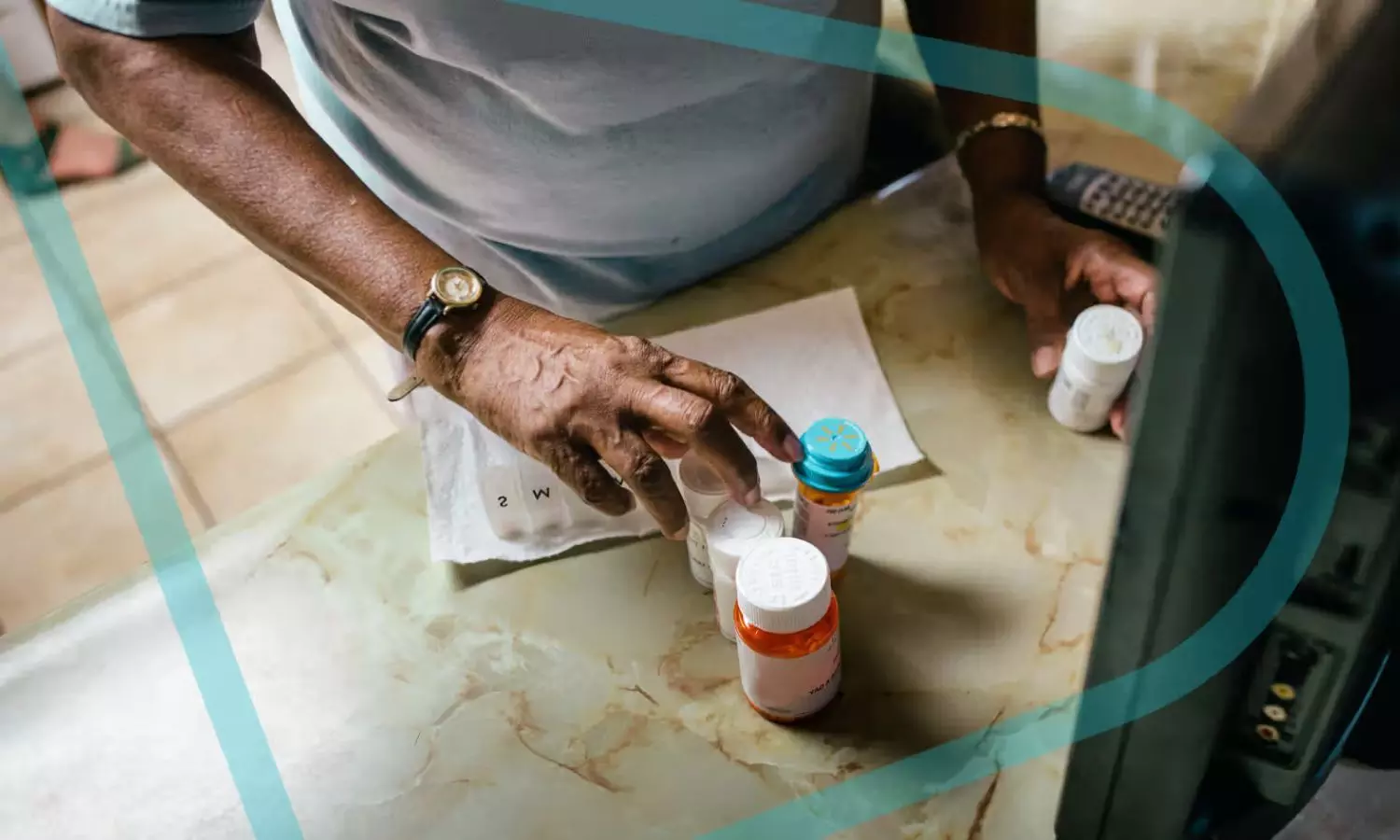
For more than five decades, the most common treatment approach for hemophilia A and B has been factor replacement therapy, which replaces missing clotting factors to facilitate proper blood coagulation. Marstacimab is a novel, investigational treatment for hemophilia that is designed to restore hemostasis by inhibiting TFPI. For appropriate patients living with hemophilia A and B, the goal of this treatment is to prevent potentially life-threatening bleeds with a once-weekly, subcutaneous flat-dose administration.
The submissions for marstacimab are based on efficacy and safety data from the Phase 3 BASIS trial (NCT03938792). Key findings were recently presented at the American Society of Hematology (ASH) Annual Meeting and Exposition on December 9, 2023. The inhibitor cohort of the BASIS trial has completed enrollment and is expected to read out as early as late 2024.
Read also: Pfizer Elrexfio gets European Commission approval for Multiple Myeloma
Powered by WPeMatico