3D elastography displays better performance in differentiating benign and malignant breast lesions
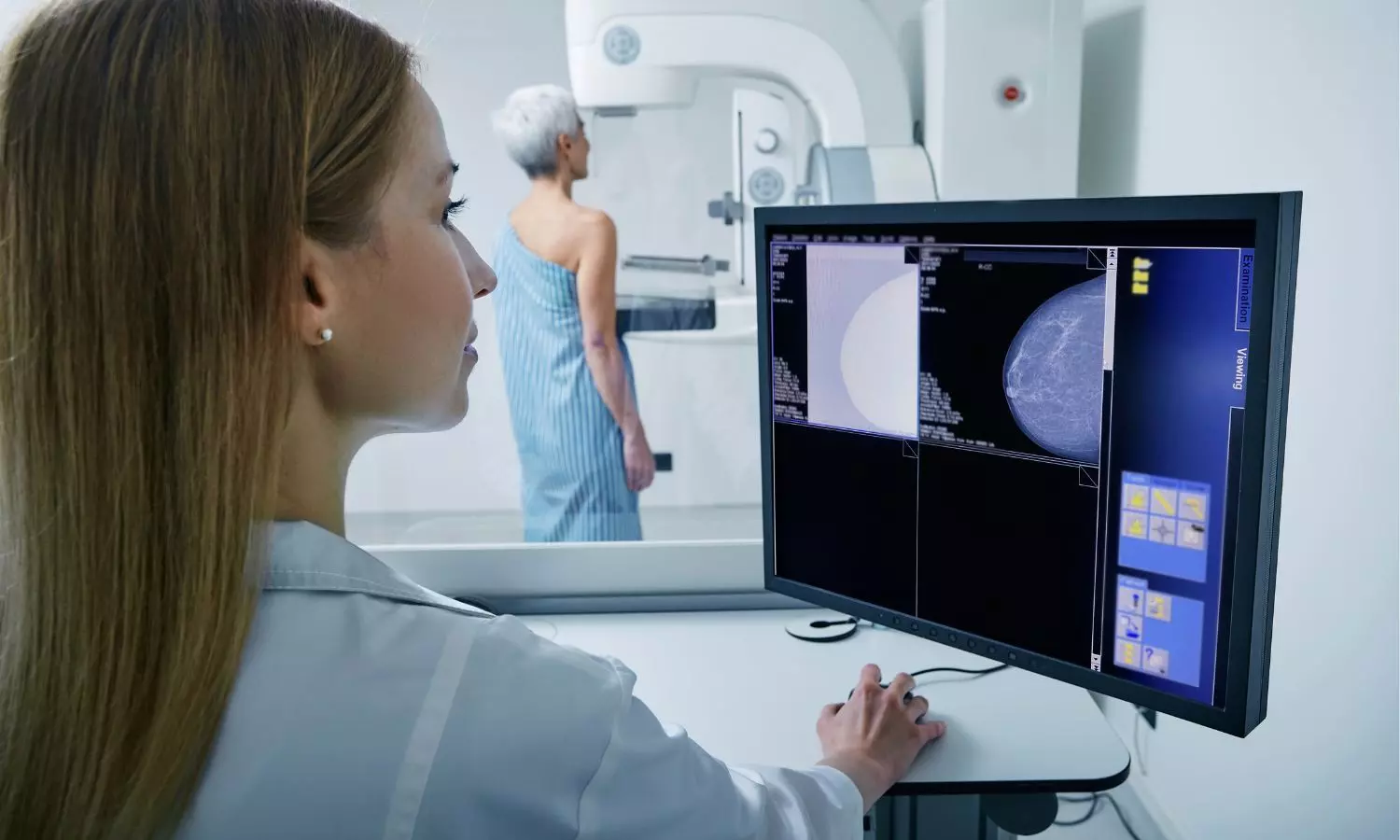
Ultrasound imaging is an alternative option for breast cancer detection, especially for women with dense breasts. However, using hand-held transducers makes screening dependent on the operator and time-consuming, leading to increased costs. Automated breast volume scanners (ABVSs) were introduced to address this issue. ABVSs use a large linear transducer to acquire ultrasound data and reconstruct 3-D images of the breast, which can be visualized in multiple planes.
In their recent study, Gijs A.G.M. Hendriks and colleagues concluded that three-dimensional strain imaging was successfully implemented on a clinically used ABVS to obtain, visualize and analyze in vivo strain images in three dimensions. Results showed significantly increased maximal principal strain ratios in malignant lesions compared to benign lesions.
This study was published in Ultrasound in Medicine and Biology.
Studies show that adding 2-D quasi-static elastography to B-mode ultrasound improves malignant lesion detection specificity. Malignant lesions are typically stiffer than benign lesions. However, this method has user dependency issues, making it unsuitable for breast screening. To overcome this, researchers implemented quasi-static elastography in an ABVS, a 3-D ultrasound system that is operator-independent and useful for screening women with dense breasts. The present study aimed to determine if 3-D quasi-static elastography in a clinically used ABVS can differentiate between benign and malignant breast lesions.
Volumetric breast ultrasound radiofrequency data sets from 82 patients were collected before and after automated transducer lifting for breast examinations. Lesions were marked, and strain was calculated using a custom algorithm. Two strain ratios were calculated for each lesion based on axial and maximal principal strain.
The study results are:
- Forty-four lesions were detected, including 9 carcinomas, 23 cysts and 12 other benign lesions.
- There was a significant difference between malignant and benign using maximal principal strain ratios.
- The axial strain ratio did not reveal a significant difference between benign and malignant lesions.
Concluding further, they said that our study results revealed that maximal principal strain ratios are increased in malignant lesions compared to benign lesions.
This research is supported by Siemens Healthineers and the Dutch Technology Foundation, which is partly funded by the Ministry of Economic Affairs, they acknowledged.
Reference:
Hendriks GAGM, et al. Automated 3-D Ultrasound Elastography of the Breast: An In Vivo Validation Study. Ultrasound Med Biol. 2023 Dec 15:S0301-5629(23)00367-8. doi: 10.1016/j.ultrasmedbio.2023.11.006. Epub ahead of print. PMID: 38103946.
Powered by WPeMatico