MP: Stray dog roams inside patient ward of Chhatarpur district hospital, probe ordered
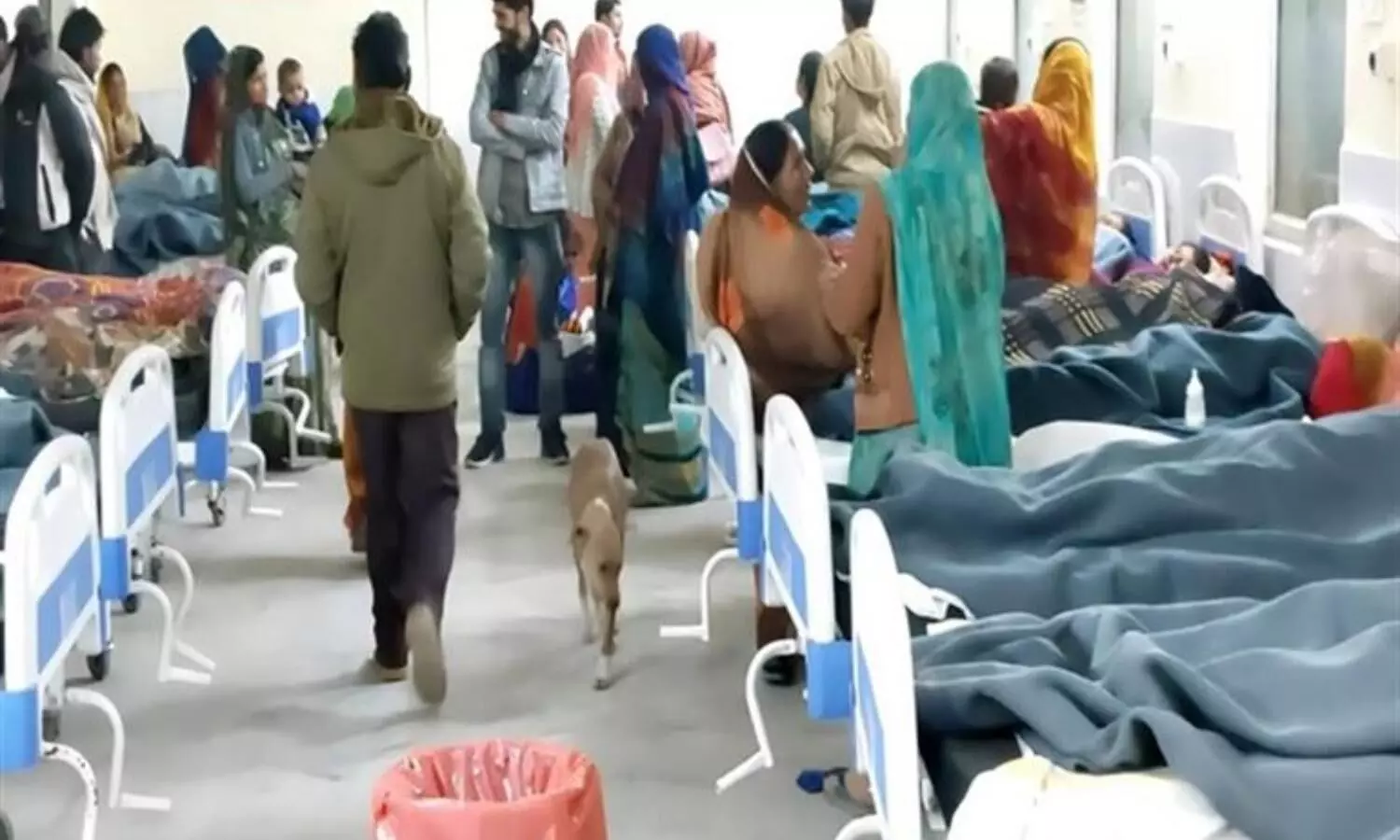
Chhatarpur: In a shocking incident, a stray dog was allegedly found roaming inside the patient ward of the Chhatarpur district hospital on Thursday. The concerned authorities are probing the mattter.
A serious negligence of the Chhatarpur district hospital management has come to light as a stray dog was found roaming inside the patient wards of the hospital on Thursday.
After the incident, the Chief Medical and Health Officer (CMHO) said that it was a serious matter and strict action would be taken against the concerned person.
“It is a very serious matter that stray dogs are roaming inside the patient ward. I will instruct the civil surgeon to take strict action against the concerned employees. There should not be such mismanagement.
It also causes infection, it may bite anyone and anything can happen. So, it is a very serious issue, I will tell the civil surgeon to make proper arrangements and ensure that this kind of carelessness should not happen again,” CMHO Lakhan Tiwari told ANI.
According to the hospital management, there has been an influx of women patients for sterilisation operations here these days and around 80 women patients were operated on Thursday.
Meanwhile, amid the sterilisation operations in the hospital, construction works are also going on the hospital due to which there is dust near the operation theatre but no prevention arrangements were made by the hospital management.
Reacting to it, CMHO Tiwari said, “There are arrangements where the operation is being conducted in the district hospital. There are no major issues of dust but the civil surgeon can make arrangements to stop it by putting curtains. The civil surgeon is being directed to fix the disorders at earliest and there should be no such carelessness again.”
Besides, an Anganwadi ASHA worker, Sangeeta Yadav also said that there was no proper cleanliness in the hospital and there was a lot of fear of the infection.
“We encourage women from our villages and bring them here for sterilisation. After the operation, we take them home and take care of them for eight days. I want to say that attention should be paid here for cleanliness and also we Asha workers and our beneficiaries are not treated properly,” She told ANI.
“There is a lot of fear of infection among the women because of dust and the construction work going on here. We all have been informed about the problems in our respective blocks but we didn’t hear back from them yet,” the ASHA worker added.
Powered by WPeMatico